桑属(Morus Linn.)植物为桑科,约16 种,其主要分布在北温带,在欧洲、南美、北美、非洲和亚洲均有广泛种植[1-2]。其品种较多,常见的品种有红桑(Morus rubra Linn.)、黑桑(Morus nigra Linn.)和白桑(Morus alba Linn.)等[3]。
桑葚(Fructus Mori)为桑科桑属的果穗,又名桑椹子、桑果、桑枣、乌椹等,长1~2 厘米,形状呈椭圆形,表面不平滑。桑葚未成熟时为绿色,成熟的过程中逐渐变为红色,成熟后为紫红色或紫黑色[4],此时食用最佳,如《本草新编》记载“紫者第一,红者次之,青者不可用”[5]。桑葚主要生长在热带、亚热带以及气候温和的地区[6]。在中国大部分地区均可种植,如江苏、浙江、湖南、四川、河北等地[7]。
桑葚中含有糖、游离酸、蛋白质、维生素和氨基酸等营养物质[8-10],具有很高的营养价值和保健功能,享有“中华果圣”之美称,并于1988年被原国家卫生部列入“药食同源”目录[8]。根据2015年版《中国药典》中的记载,桑葚气微,味微酸而甜,用于主治“滋阴补血,生津润燥,肝肾阴虚,眩晕耳鸣,心悸失眠,须发早白,津伤口渴,内热消渴,肠燥便秘”[4]。现代医学研究表明,桑葚中的活性物质主要是多糖和多酚[9-11],具有补肝益肾、润肠通便、抗衰老、降糖降脂等药理作用[12-16]。
多糖是一种广泛存在于植物、动物和微生物中的生物大分子[17]。由于天然多糖具有重要的生物活性,且拥有相对较低的毒性,人们对其提升人体健康功效的兴趣与日俱增[18]。桑葚多糖作为桑葚水提物的主要成分之一,具有重要的生物活性。许多研究表明,桑葚多糖主要是一种果胶型多糖[19-21],具有良好的抗氧化、降血糖、免疫调节和调节肠道菌群等作用[20,22-24]。本文根据国内外综述报道,重点对桑葚多糖的分离纯化、结构特征及生物活性进行系统归纳总结,为桑葚多糖的后续研究与开发利用提供新的思路和技术参考。

图1 桑葚的新鲜果实(a)和桑葚干(b)
Fig.1 Fresh Fructus Mori (a) and dried Fructus Mori (b)
1 桑葚多糖的提取
多糖是一种天然的极性大分子,根据“相似相溶”原理,在提取过程中一般采用极性溶剂进行提取,如热水、稀酸、稀碱溶液等[25],有时也会采用一些辅助手段提取,包括酶法、高压、微波、超声等[26-29]。桑葚多糖的提取方法主要为水提醇沉法、热水浸提法、超声辅助提取法、酶法辅助提取法等[30-33],不同的提取方法对所得多糖的结构和理化性质有不同的影响。桑葚多糖的提取方法见表1。
由表1可知,桑葚多糖多采用水提醇沉法提取,提取温度一般选取70~90 ℃。据报道,桑葚多糖的得率最高虽可达17.78%,但其波动较大(3.01%~17.78%),而这可能与桑葚的品种、产地和提取方法有关,如Wang 等[22]采用水提醇沉法进行响应面优化试验,其最佳提取条件为提取温度87 ℃、提取时间3 h、液料比39∶1,提取率为11.86%。然而,多糖得率也存在较低的情况,如江岩等[8]采用正交试验优化桑葚多糖提取工艺,其最佳提取条件为浸提时间2 h、料液比30∶1、浸提温度70℃、浸提3 次,在此条件下多糖得率仅为3.24%。许多研究报道了采用不同的溶剂和提取方法依次提取,以期提高多糖得率[41-42],然而不同的提取方法对多糖的充分提取有着很大的影响。Chen 等[43]比较了热水浸提法、酶辅助浸提法、超声辅助浸提法和酶-超声辅助浸提法分别提取山楂多糖,其多糖得率分别为5.88%,7.47%,9.59%和10.39%,其中传统的热水浸提法多糖得率最低,而采用了辅助提取方法的得率有了明显升高。此外,He 等[44]比较不同的提取方法提取铁皮石斛茎中多糖也有相同的发现。因此,可以采用一些辅助提取手段提高桑葚多糖的得率,在桑葚多糖提取工艺中采用酶法辅助提取进行响应面优化试验可使多糖得率达到16.16%[39]。此外,由于桑葚多糖多报道为果胶型多糖,而果胶适用于酸性介质提取[45-46],因此可以根据果胶的性质采用酸提法从桑葚中提取多糖,达到提高桑葚果胶多糖的得率的效果。
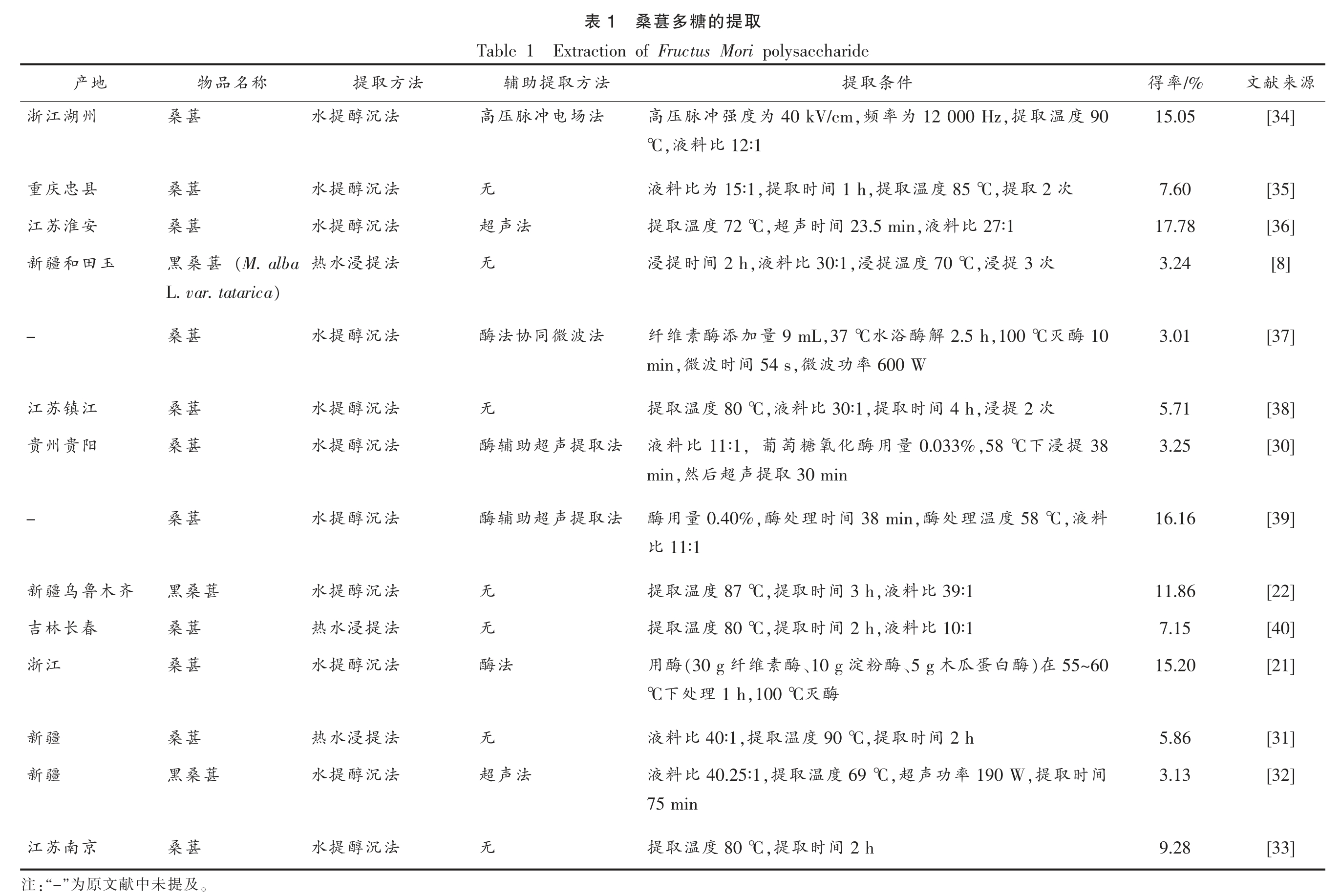
源来献[34][35][36][8][37][38][30][39][22][40][21][31][32][33]文/%率得15.05 7.60 17.78 3.24 3.01 5.71 3.25 16.16 11.86 7.15 15.20 5.86 3.13 9.28取提的糖多葚桑1表Extraction of Fructus Mori polysaccharide Table 1 件条取提法方取提助辅90度温取12 000 Hz,提为率40 kV/cm,频为度强冲脉压高法场电冲脉压高12∶1比料,液℃2 次取85 ℃,提度温取1 h,提间时取15∶1,提为比料液无27∶1比料,液23.5 min间时声,超72 ℃度温取提法声超次3提,浸70 ℃度温提,浸30∶1比料,液2 h间时提浸无10酶灭,100 ℃2.5 h解酶浴水,37 ℃9 mL量加添酶素维纤法波微同协法酶600 W率功波,微54 s间时波,微min次2提,浸4 h间时取,提30∶1比料,液80 ℃度温取提无38提浸下,58 ℃0.033%量用酶化氧糖萄,葡11∶1比料液法取提声超助辅酶30 min取提声超后,然min料,液58 ℃度温理处,酶38 min间时理处,酶0.40%量用酶法取提声超助辅酶11∶1比39∶1比料,液3 h间时取,提87 ℃度温取提无10∶1比料2 h,液间时取80 ℃,提度温取提无55~60)在酶白蛋瓜木、5 g酶粉淀、10 g酶素维纤(30 g酶用法酶酶1 h,100 ℃灭理处℃下2 h间时取,提90 ℃度温取,提40∶1比料液无间时取,提190 W率功声,超69 ℃度温取,提40.25∶1比料液法声超75 min 2 h间时取,提80 ℃度温取提无法方取提法沉醇法沉醇法沉醇法提浸法沉醇法沉醇法沉醇法沉醇法沉醇法提浸法沉醇法提浸法沉醇法沉醇提水提水提水水热提水提水提水提水提水水热提水水热提水提水称名品物葚桑葚桑葚桑(M.alba葚桑黑)L.var.tatarica葚桑葚桑葚桑葚桑葚桑黑葚桑葚桑葚桑葚桑黑葚桑。及提未中地产州湖江浙县忠庆重安淮苏江玉田和疆新-江镇苏江阳贵州贵-齐木鲁乌疆新春长林吉江浙疆新疆新京南苏江献文原”为:“-注
2 桑葚多糖的纯化
经提取得到的桑葚多糖粗提液,常含有非多糖成分,如大分子蛋白质和小分子色素等物质[47]。为了制备高纯度的多糖,可除去多糖粗提液中非多糖成分,从而富集多糖成分。除蛋白质常用的方法有Sevag 法、三氯乙酸法、酶法等[48-50]。由表2可知,Sevag 法是桑葚多糖常用的除蛋白法,具有操作简便,蛋白易于去除的优点[51],然而该方法效率不高,且会造成多糖损失[47]。

参考文献[59][22][60][56][57][23][33][20][61]化纯离分的糖多葚桑2表Isolation and purification of Fructus mori polysaccharide Table 2 化纯离分质性化理分组化纯分组得所化纯法析层柱胶凝法换交子离-、、T3-2 T3-1-DEAE-cellulose-52、T3-4 T3-3 column;到测检未白、蛋13.26%酸醛、糖83.01%量含:糖BMP-1-1、BMP-1-1 G-Sephadex DEAE-cellulose-52到测检未白、蛋29.71%酸醛、糖88.15%量含:糖BMP-2-1 BMP-2-1(1.6 100 column(2.6 cm × 60 column)cm×100 cm)cm-、MFPMFP-1 G-Sephadex DEAE-cellulose-52、MFP-3 2(1 100 column(3.2 cm × 30 column)cm×1.5 m)cm 3.32%;白47.4%、蛋酸醛糖乳58.97%、半量含MFP-1:糖MFP-1、MFP-30%,60%和用-3.21%;白10.9%、蛋酸醛糖乳62.18%、半量含MFP-2:糖2、MFP-3分醇乙的90%3.83%白2.9%、蛋酸醛糖乳55.76%、半量含MFP-3:糖沉醇级到测检未白、蛋5.4%酸醛糖乳、半95.4%量含糖MFP4P G-Sephadex-Sepharose DEAE(3.5 100 column(2.6 fast flow column)cm × 50 cm)cm × 40 cm;3.54%白、蛋1.57%酸醛、糖88.37%量含:糖MFP-1、MFPMFP-1--Sepharose DEAE;6.31%白、蛋2.11%酸醛、糖75.68%量含:糖MFP-2、、MFP-3 2(2.6 fast flow column;5.74%白、蛋6.18%酸醛、糖86.34%量含:糖MFP-3 MFP-4)cm× 40 cm 4.11%白、蛋31.9%酸醛、糖81.33%量含:糖MFP-4-MFP3P G-Sephadex-Sepharose DEAE(3.5 100 column(2.6 fast flow column)cm × 50 cm)cm × 40 cm 0.30%白、蛋11.25%酸醛、糖46.51%量含糖JS-MP-1-DEAE-cellulose col-)(3 cm×45 cm umn;0.13%白、蛋50.69%酸醛糖乳、半92.81%量含:糖MFP1、、MFP2 MFP1-DEAE-cellulose-52;0.49%白、蛋71.55%酸醛糖乳、半95.26%量含:糖MFP2 MFP3 column 0.96%白、蛋49.12%酸醛糖乳、半85.09%量含:糖MFP3。及提未中献文”为;“-法方化纯法附吸----大AB-8脂树孔大AB-8脂树孔大AB-8脂树孔-大AB-8脂树孔离分用使法白蛋脱盐ZnSO4法析-法Sevag Sevag 法法Sevag法Sevag法Sevag-法Sevag序顺按糖多葚品物称名葚桑葚桑黑葚桑葚桑葚桑葚桑黑葚桑葚桑葚桑黑桑为右到地产州广东广疆新阳贵州贵江浙疆新疆新疆新国韩疆新左从格:表注
桑葚多糖粗提液脱蛋白后通常还含有色素,影响多糖的分析和测定,因此需要对桑葚粗提液进行脱色。目前常用的脱色法方有离子交换法、氧化法和吸附法等[52-54]。由于氧化脱色具有暂时性,一旦有还原剂的加入,多糖溶液中的色素物质就会显现出原来的颜色[55],所以桑葚多糖目前多采用大孔树脂吸附法和离子交换法进行脱色,其中离子交换法具有诸多优点:脱色容量高,能除去色素等阴离子物质或弱极性物质,同时还能分离中性糖与酸性糖[47],因此常用于桑葚多糖的脱色。
由表2可知,桑葚多糖常用的纯化方法包括分级醇沉法、离子交换柱层析法和凝胶柱层析法等。如Hu 等[56]利用分级醇沉法纯化桑葚多糖,可得到3 个纯化组分MFP-1 (30%乙醇沉淀物)、MFP-2(60%乙醇沉淀物)和MFP-3(90%乙醇沉淀物)。为了达到更好的纯化效果,往往需要综合利用几种纯化方法,比如离子交换柱层析和凝胶柱层析法是纯化桑葚多糖最常用的结合方法,能够有效地纯化桑葚多糖,如Wang 等[57]结合DEAE-Sepharose Fast Flow 柱和Sephadex G-100凝胶层析柱纯化出MFP-4 桑葚多糖组分,其多糖含量为95.4%。桑葚粗多糖通过分离纯化后,可获得相对均一的多糖组分,结合高效液相色谱对组分纯度的鉴定,可进一步对多糖结构进行表征[58],再结合甲基化和核磁共振等对各组分结构进行解析。
3 桑葚多糖的基本结构特征
桑葚多糖的组成通常是几种单组分构成的多糖复合物,根据不同提取方法所得多糖的单糖组成也存在差异,一般认为桑葚多糖为阿拉伯糖、鼠李糖、半乳糖、葡萄糖和半乳糖醛酸等单糖组成[60,62-63]。由表3可知,桑葚多糖的分子质量普遍集中在80~250 ku,然而其分子质量在不同文献报道中差异较大:最低为0.7 ku,最高达1 639 ku,如Lee 等[20]用DEAE-Cellulose 柱纯化得到的桑葚Oddi 多糖组分JS-MP-1,测得其分子质量为1 639 ku,远高于其它文献报道的结果。
此外,部分桑葚多糖中鼠李糖为主要成分。Chen 等[31]使用DEAE-Sepharose Fast Flow 柱对桑葚粗多糖溶液进行处理得到MFP-3 组分,再用Sephadex G-100 凝胶层析柱纯化得到MFP3P,平均分子质量为136.6 ku,其单糖组成为鼠李糖(25.98%)、半乳糖(23.1%)、阿拉伯糖(21.51% )、半乳糖醛酸(16.35%)、葡糖糖(13.06%),其中鼠李糖占比达25.98%。Li 等[21]采用DEAE-Sepharose Fast Flow 柱和Sephacryl S-300 HR 凝胶层析柱纯化得到的FMP-6-S2,该组分单糖主要由鼠李糖(30.91%)、半乳糖醛酸(24.78%)、半乳糖(28.70%)和阿拉伯糖(15.61%)组成,这两个组分可能为鼠李半乳糖醛酸聚糖(RG-I 型)果胶和阿拉伯半乳聚糖(AG型) 果胶。RG-I 型果胶主要由α-D-GalpA 构成,同时还含有α-LRhap、α-L-Araf 和β-D-Galp 等中性糖[64],而AG 型由1,4 连接的β-D-Galp 主链组成,含有α-L-Araf 残基的特点[65]。以上桑葚多糖中鼠李糖、半乳糖醛酸、阿拉伯糖和半乳糖为主要单糖,为典型的RG-I 型果胶和AG 型果胶侧链结构。因此,在桑葚多糖结构的表征过程中可以结合RG-I 型果胶和AG 型果胶的特点来解析。
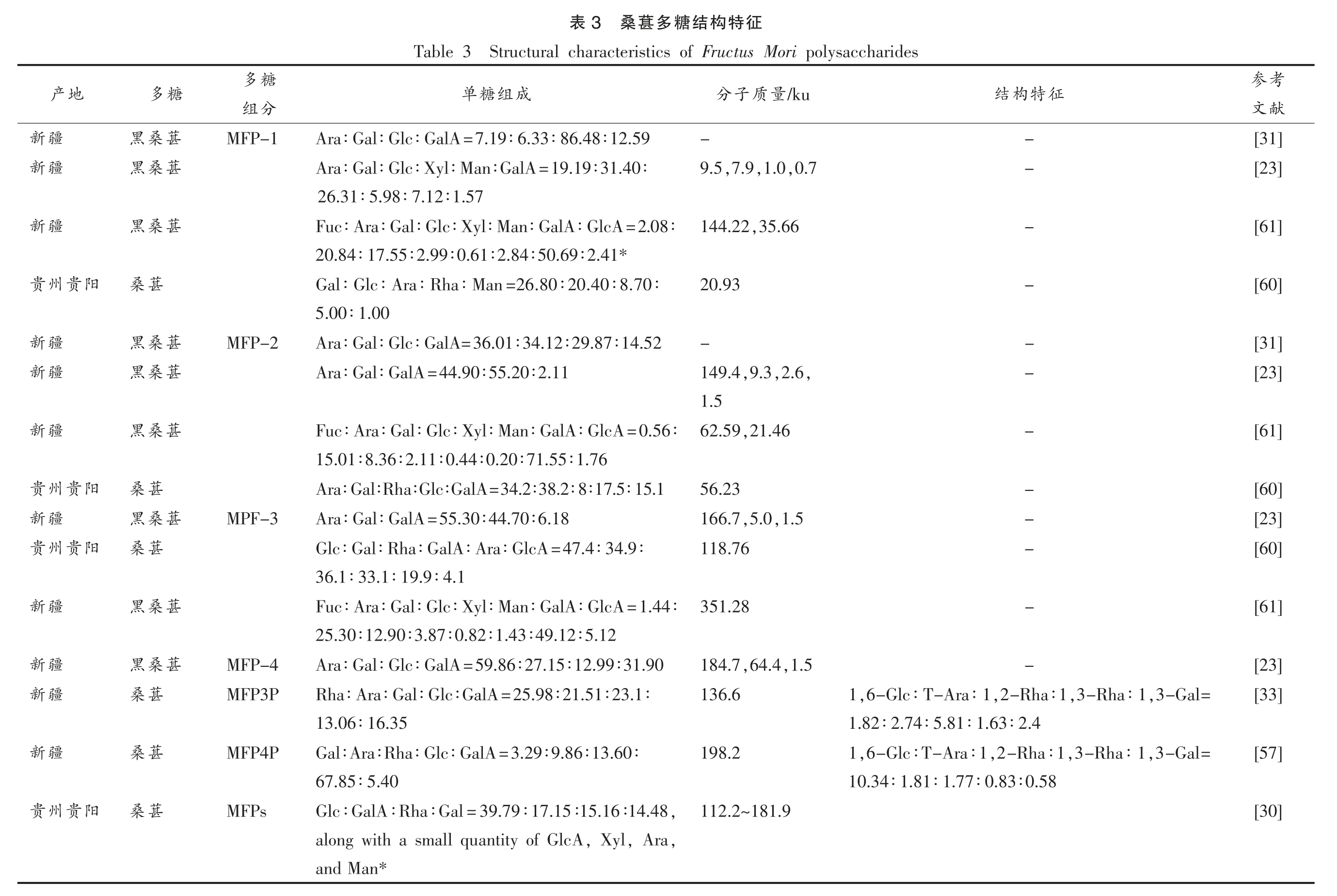
考参献文[31][23][61][60][31][23][61][60][23][60][61][23][33][57][30]征特构结糖多葚桑3表Structural characteristics of Fructus Mori polysaccharides Table 3 征特构结/ku量质子分成组糖单--Ara∶Gal∶Glc∶GalA=7.19∶6.33∶86.48∶12.59-9.5,7.9,1.0,0.7 Ara∶Gal∶Glc∶Xyl∶Man∶GalA=19.19∶31.40∶26.31∶5.98∶7.12∶1.57-,35.66 144.22 Fuc∶Ara∶Gal∶Glc∶Xyl∶Man∶GalA∶GlcA=2.08∶20.84∶17.55∶2.99∶0.61∶2.84∶50.69∶2.41*-20.93 Gal∶Glc∶Ara∶Rha∶Man=26.80∶20.40∶8.70∶5.00∶1.00--Ara∶Gal∶Glc∶GalA=36.01∶34.12∶29.87∶14.52-149.4,9.3,2.6,Ara∶Gal∶GalA=44.90∶55.20∶2.11 1.5-,21.46 62.59 Fuc∶Ara∶Gal∶Glc∶Xyl∶Man∶GalA∶GlcA=0.56∶15.01∶8.36∶2.11∶0.44∶0.20∶71.55∶1.76-56.23 Ara∶Gal∶Rha∶Glc∶GalA=34.2∶38.2∶8∶17.5∶15.1-,1.5,5.0 166.7 Ara∶Gal∶GalA=55.30∶44.70∶6.18-118.76 Glc∶Gal∶Rha∶GalA∶Ara∶GlcA=47.4∶34.9∶36.1∶33.1∶19.9∶4.1-351.28 Fuc∶Ara∶Gal∶Glc∶Xyl∶Man∶GalA∶GlcA=1.44∶25.30∶12.90∶3.87∶0.82∶1.43∶49.12∶5.12-,1.5,64.4 184.7 Ara∶Gal∶Glc∶GalA=59.86∶27.15∶12.99∶31.90,3-Gal=,3-Rha∶1,2-Rha∶1,6-Glc∶T-Ara∶1 1 136.6 Rha∶Ara∶Gal∶Glc∶GalA=25.98∶21.51∶23.1∶1.82∶2.74∶5.81∶1.63∶2.4 13.06∶16.35 1,6-Glc∶T-Ara∶1,2-Rha∶1,3-Rha∶1,3-Gal=198.2 Gal∶Ara∶Rha∶Glc∶GalA=3.29∶9.86∶13.60∶10.34∶1.81∶1.77∶0.83∶0.58 67.85∶5.40 112.2~181.9,Glc∶GalA∶Rha∶Gal=39.79∶17.15∶15.16∶14.48,,Ara,Xyl along with a small quantity of GlcA and Man*糖多分组MFP-1 MFP-2 MPF-3 MFP-4 MFP3P MFP4P MFPs糖葚葚葚葚葚葚葚葚葚多桑桑桑葚桑桑桑葚桑葚桑桑葚葚葚黑黑黑桑黑黑黑桑黑桑黑黑桑桑桑地产疆疆疆阳贵州疆疆疆阳贵州疆阳贵州疆疆疆疆阳贵州新新新贵新新新贵新贵新新新新贵
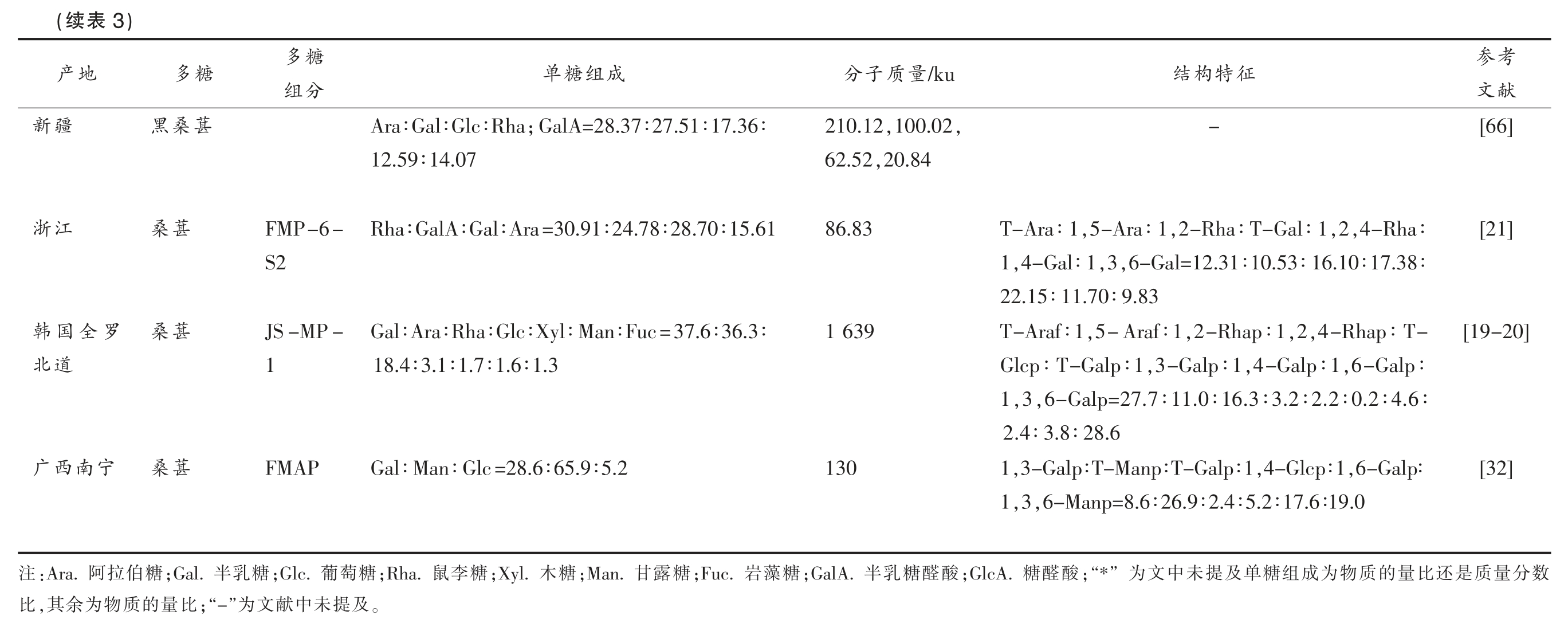
考参征特构结/ku量质子分献文[66]-,,100.02 210.12,20.84 62.52[21],4-Rha∶,2,2-Rha∶T-Gal∶1,5-Ara∶1 T-Ara∶1 86.83,6-Gal=12.31∶10.53∶16.10∶17.38∶,3,4-Gal∶1 1 22.15∶11.70∶9.83[19-20],4-Rhap∶T-,2,2-Rhap∶1,5-Araf∶1 T-Araf∶1 1 639,6-Galp∶,4-Galp∶1,3-Galp∶1 Glcp∶T-Galp∶1,6-Galp=27.7∶11.0∶16.3∶3.2∶2.2∶0.2∶4.6∶,3 1 2.4∶3.8∶28.6[32]1,3-Galp∶T-Manp∶T-Galp∶1,4-Glcp∶1,6-Galp∶130 1,3,6-Manp=8.6∶26.9∶2.4∶5.2∶17.6∶19.0数分量质是还比量的质物为成组糖单及提未中文” 为;“*酸醛糖;GlcA.酸醛糖乳半;GalA.3)表(续糖多成组糖单糖多地产分组;GalA=28.37∶27.51∶17.36∶Ara∶Gal∶Glc∶Rha葚桑黑疆新12.59∶14.07 Rha∶GalA∶Gal∶Ara=30.91∶24.78∶28.70∶15.61 FMP-6-葚桑江浙S2 Gal∶Ara∶Rha∶Glc∶Xyl∶Man∶Fuc=37.6∶36.3∶JS-MP-葚桑罗全国韩18.4∶3.1∶1.7∶1.6∶1.3 1道北Gal∶Man∶Glc=28.6∶65.9∶5.2 FMAP 葚桑宁南西广糖藻岩;Fuc.糖露甘;Man.糖木;Xyl.糖李鼠;Rha.糖萄葡;Glc.糖乳半;Gal.糖伯拉阿:Ara.注。及提未中献文”为;“-比量的质物为余,其比
目前,国内外对于桑葚多糖的结构研究相对较少,解析得到的多糖多为果胶型多糖,其结构示意图见图2和图3。部分多糖的结构为由(1→3)连接的甘露聚糖组成,主链甘露糖基残基上的半乳糖基和葡萄糖基残基在O-6 处,结构示意图见图4。
在2007年,Wei 等[32]首先报道了一种通过水提醇沉法提取的水溶性桑葚多糖FMAP(图4),由Gal、Man 和Glc 等单糖组成,具有β-吡喃糖苷键,并且多糖的主链由(1→3)连接的甘露聚糖组成,半乳糖基和葡萄糖基残基的分支附着在O-6 上。然而,近些年对桑葚多糖结构解析的研究发现,桑葚多糖多为果胶多糖,如Li 等[21]解析了桑葚多糖组分FMP-6-S2 的化学结构(图2),该组分主链为→2)-α-Rhap-(1→4)-α-GalpA-(1→组成的RG-I 型结构,并且在α-Rhap 的O-4 发生侧链取代;侧链R1 为→5)-α-Araf-(1→组成,R2 为→4)-α-Galp-(1→或末端为1,3,6-连接的β-Galp 和→4)-α-Galp-(1→组成。此外,Lee 等[20]通过乙醇沉淀和DEAE纤维素离子交换色谱分离和纯化了一种果胶多糖JS-MP-1(图3)主要由Gal、Ara 和Rha 组成,是一种酸性杂多糖,很可能是支链RG-I 果胶多糖的变体,JS-MP-1 的中性糖侧链为→5)-α-L-Araf-(1→和具有→6)-β-D-Galp-(1→和末端为3-O-结合α-L-Araf 的支链阿拉伯半乳聚糖II 型(AGII)。阿拉伯糖链在一些α-L-Rhap 单元的O-4 位置与RG-I 主链连接。然而,RG-I 和AG-II 链之间的连接模式尚不清楚[19]。以上分析表明桑葚多糖结构复杂,除RG-I、AG-II、阿拉伯聚糖等果胶中常见结构类型外,还含有半乳甘露聚糖结构。

图2 桑葚水提多糖FMP-6-S2 结构示意图[21]
Fig.2 Structural schematic diagram of Fructus Mori water-extracted polysaccharide FMP-6-S2[21]

图3 桑葚水提多糖JS-MP-1 结构示意图[19]
Fig.3 Structural schematic diagram of Fructus Mori water-extracted polysaccharide JS-MP-1[19]

图4 桑葚水提多糖FMAP 结构示意图[32]
Fig.4 Structural schematic diagram of Fructus Mori water-extracted polysaccharide FMAP[32]
4 桑葚多糖的生物活性
目前,桑葚多糖在体外试验、动物实验和细胞培养试验中表现出免疫调节、降血糖、抗氧化、调节肠道菌群等多种生理功能,其生理功能的研究对于促进桑葚多糖在功能性食品中的应用具有重大意义,具体活性功能及作用方式见表4。
表4 桑葚多糖的生物活性
Table 4 Biological activities of Fructus Mori polysaccharides
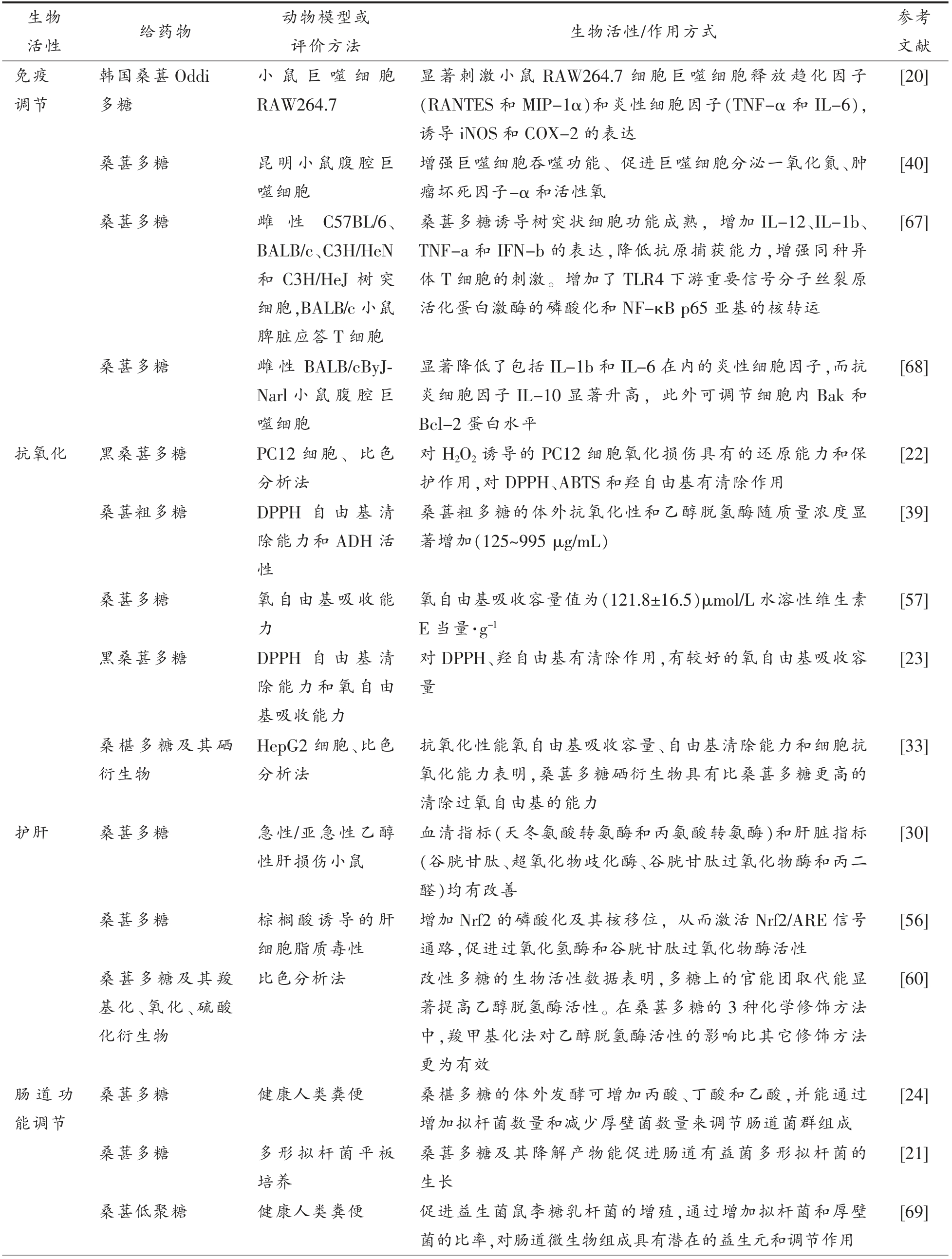
评价方法 生物活性/作用方式 参考文献免疫调节活性 给药物 动物模型或生物韩国桑葚Oddi多糖小鼠巨噬细胞RAW264.7显著刺激小鼠RAW264.7 细胞巨噬细胞释放趋化因子(RANTES 和MIP-1α)和炎性细胞因子(TNF-α 和IL-6),诱导iNOS 和COX-2 的表达[20]桑葚多糖 昆明小鼠腹腔巨噬细胞桑葚多糖 雌 性 C57BL/6、BALB/c、C3H/HeN和C3H/HeJ 树突细胞,BALB/c 小鼠脾脏应答T 细胞桑葚多糖 雌性BALB/cByJNarl 小鼠腹腔巨噬细胞抗氧化 黑桑葚多糖 PC12 细胞、比色分析法桑葚粗多糖 DPPH 自 由基 清除能力和ADH 活性桑葚多糖 氧自由基吸收能力黑桑葚多糖 DPPH 自 由基 清除能力和氧自由基吸收能力增强巨噬细胞吞噬功能、促进巨噬细胞分泌一氧化氮、肿瘤坏死因子-α 和活性氧桑葚多糖诱导树突状细胞功能成熟,增加IL-12、IL-1b、TNF-a 和IFN-b 的表达,降低抗原捕获能力,增强同种异体T 细胞的刺激。增加了TLR4 下游重要信号分子丝裂原活化蛋白激酶的磷酸化和NF-κB p65 亚基的核转运显著降低了包括IL-1b 和IL-6 在内的炎性细胞因子,而抗炎细胞因子IL-10 显著升高,此外可调节细胞内Bak 和Bcl-2 蛋白水平对H2O2 诱导的PC12 细胞氧化损伤具有的还原能力和保护作用,对DPPH、ABTS 和羟自由基有清除作用桑葚粗多糖的体外抗氧化性和乙醇脱氢酶随质量浓度显著增加(125~995 μg/mL)氧自由基吸收容量值为(121.8±16.5)μmol/L 水溶性维生素E 当量·g-1对DPPH、羟自由基有清除作用,有较好的氧自由基吸收容量[40][67][68][22][39][57][23]桑椹多糖及其硒衍生物HepG2 细胞、比色分析法抗氧化性能氧自由基吸收容量、自由基清除能力和细胞抗氧化能力表明,桑葚多糖硒衍生物具有比桑葚多糖更高的清除过氧自由基的能力[33]护肝 桑葚多糖 急性/亚急性乙醇性肝损伤小鼠桑葚多糖 棕榈酸诱导的肝细胞脂质毒性血清指标(天冬氨酸转氨酶和丙氨酸转氨酶)和肝脏指标(谷胱甘肽、超氧化物歧化酶、谷胱甘肽过氧化物酶和丙二醛)均有改善增加Nrf2 的磷酸化及其核移位,从而激活Nrf2/ARE 信号通路,促进过氧化氢酶和谷胱甘肽过氧化物酶活性[30][56]桑葚多糖及其羧基化、氧化、硫酸化衍生物比色分析法 改性多糖的生物活性数据表明,多糖上的官能团取代能显著提高乙醇脱氢酶活性。在桑葚多糖的3 种化学修饰方法中,羧甲基化法对乙醇脱氢酶活性的影响比其它修饰方法更为有效[60]肠道功能调节桑葚多糖 健康人类粪便 桑椹多糖的体外发酵可增加丙酸、丁酸和乙酸,并能通过增加拟杆菌数量和减少厚壁菌数量来调节肠道菌群组成[24]桑葚多糖 多形拟杆菌平板培养桑葚多糖及其降解产物能促进肠道有益菌多形拟杆菌的生长[21]桑葚低聚糖 健康人类粪便 促进益生菌鼠李糖乳杆菌的增殖,通过增加拟杆菌和厚壁菌的比率,对肠道微生物组成具有潜在的益生元和调节作用[69]
(续表4)
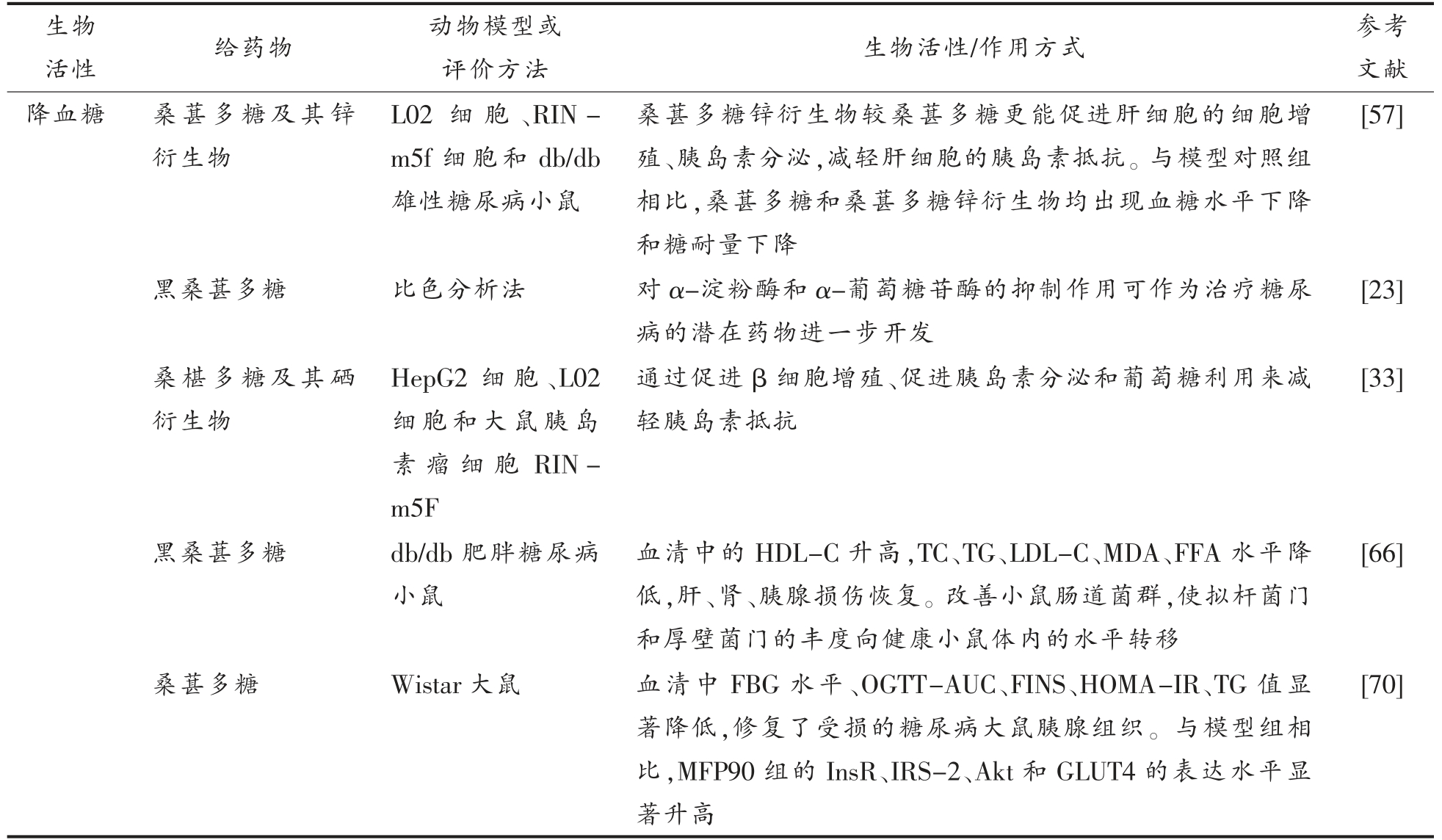
评价方法 生物活性/作用方式 参考文献降血糖 桑葚多糖及其锌衍生物活性 给药物 动物模型或生物L02 细 胞、RIN-m5f 细胞和db/db雄性糖尿病小鼠桑葚多糖锌衍生物较桑葚多糖更能促进肝细胞的细胞增殖、胰岛素分泌,减轻肝细胞的胰岛素抵抗。与模型对照组相比,桑葚多糖和桑葚多糖锌衍生物均出现血糖水平下降和糖耐量下降[57]黑桑葚多糖 比色分析法 对α-淀粉酶和α-葡萄糖苷酶的抑制作用可作为治疗糖尿病的潜在药物进一步开发[23]桑椹多糖及其硒衍生物HepG2 细 胞、L02细胞和大鼠胰岛素瘤细胞RINm5F通过促进β 细胞增殖、促进胰岛素分泌和葡萄糖利用来减轻胰岛素抵抗[33]黑桑葚多糖 db/db 肥胖糖尿病小鼠血清中的HDL-C 升高,TC、TG、LDL-C、MDA、FFA 水平降低,肝、肾、胰腺损伤恢复。改善小鼠肠道菌群,使拟杆菌门和厚壁菌门的丰度向健康小鼠体内的水平转移[66]桑葚多糖 Wistar 大鼠 血清中FBG 水平、OGTT-AUC、FINS、HOMA-IR、TG 值显著降低,修复了受损的糖尿病大鼠胰腺组织。与模型组相比,MFP90 组的InsR、IRS-2、Akt 和GLUT4 的表达水平显著升高[70]
4.1 免疫调节作用
免疫调节是桑葚多糖的重要的功能活性之一,其通过多个途径对免疫系统发挥作用。在自然界中,植物、真菌和藻类中的一些多糖均具有免疫调节的作用[71-74]。多糖的免疫活性主要通过自然杀伤细胞、T 细胞、B 细胞和巨噬细胞来发挥作用[75-76]。巨噬细胞是单核吞噬细胞系统的一个重要成员,在免疫应答过程中表现出多种功能,是抵抗病原体侵入人体的第一步[77]。桑葚多糖能够增强昆明小鼠腹腔巨噬细胞的吞噬功能、促进巨噬细胞分泌一氧化氮、肿瘤坏死因子和活性氧[40]。此外,还可以刺激小鼠RAW264.7 巨噬细胞释放趋化因子(RANTES 和MIP-1α)和促炎细胞因子(TNF-α 和IL-6),诱导诱导一氧化氮合酶(iNOS)和环氧化酶(COX-2)的表达,发挥免疫调节作用[20]。
树突状细胞是强大的抗原呈递细胞,能够启动大多数免疫反应[78]。如图5所示,桑葚多糖可增加TLR4 下游重要信号分子丝裂原活化蛋白激酶的磷酸化和NF-κB p65 亚基的核转运,通过TLR4 诱导树突状细胞成熟[67]。成熟的树突状细胞可通过IL-12 激活自然杀伤细胞,从而启动免疫反应[79]。免疫反应包括非特异性免疫反应和特异性免疫反应,特异性免疫反应与T 淋巴细胞有关,其功能在初始抗原刺激后立即激活[80]。在C57BL/6小鼠源性的树突细胞和BALB/c 小鼠源性T 细胞诱导混合白细胞反应中,经桑葚多糖处理的成熟树突细胞能够有效地刺激同种异体T 细胞[67]。另一项研究发现,桑葚多糖可调节LPS 刺激的巨噬细胞细胞内Bak 和Bcl-2 蛋白水平,从而保护巨噬细胞不发生凋亡[68]。这些结果表明桑椹多糖作为免疫治疗佐剂和有益的免疫调节保健食品具有潜在的应用前景。
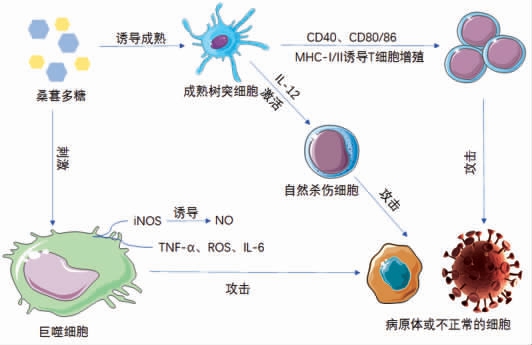
图5 桑葚多糖免疫调节机理图
Fig.5 A diagram of the immunomodulatory mechanism of Fructus Mori polysaccharides
4.2 降血糖
植物多糖促进胰岛增殖刺激胰岛细胞分泌胰岛素可能是其作用机制之一[81]。胰岛素信号通路在2 型糖尿病的发病机制中起着至关重要的作用,而胰岛素受体底物在胰岛素信号通路中起着关键作用[82-83]。在给予糖尿病小鼠400 mg/kg 桑葚多糖时,可显著改善糖尿病小鼠症状,修复受损的糖尿病大鼠胰腺组织,显著上调IRS-2 和AKT 的表达,同时上调GLUT4 的表达,起到葡萄糖代谢的限速步骤[70]。桑椹多糖通过抑制糖尿病大鼠胰岛细胞凋亡和改善胰岛素分泌能力而具有降血糖作用,并通过调节肠道微生物群改善靶向葡萄糖代谢[84]。通过研究桑椹多糖对糖尿病db/db 小鼠治疗作用,发现桑葚多糖能改善小鼠肠道菌群,使拟杆菌门和厚壁菌门的丰度向健康小鼠体内的水平转移,达到降血糖的作用[66]。对桑葚多糖改性后,桑葚多糖锌衍生物较桑葚多糖更能促进胰腺细胞增殖、增加葡萄糖消耗和胰岛素分泌,减轻肝细胞的胰岛素抵抗[57]。
4.3 抗氧化作用
氧化会导致多种疾病,包括糖尿病、老年痴呆症和心脏病[85-87],天然多糖的抗氧化活性一直是研究的焦点。多糖可通过抑制体内活性氧的和自由基产生,提高抗氧化酶的活性,促进超氧化物歧化酶的释放,从而增强机体对自由基的清除能力和抗氧化能力。桑葚多糖作为有效的还原剂、自由基清除剂和体外亚铁螯合剂,引起了人们越来越多的兴趣[1,23,61]。由表4可知,桑葚多糖具有良好的抗氧化作用,主要体现在对DPPH、羟自由基有清除作用,同时有较好的氧自由基吸收容量[23]。细胞试验表明,桑葚多糖对H2O2 诱导的PC12 细胞氧化损伤具有还原能力和保护作用,对DPPH、ABTS和羟自由基有清除作用[22]。此外,桑葚多糖的抗氧化能力还可能与其结构有关,桑葚多糖硒衍生物具有比桑葚多糖更好的抗氧化性能,氧自由基吸收容量、自由基清除能力和细胞抗氧化能力,表明桑葚多糖硒衍生物具有更高的清除过氧自由基的能力[33]。
4.4 调解肠道菌群及其它作用
除上述生理活性外,桑葚多糖还可以通过增加有益菌群(类杆菌)和减少有害菌群(厚壁菌)来调节肠道微生物群,具有潜在的益生元作用[24]。在肥胖糖尿病小鼠中,桑葚多糖可富集类杆菌、乳酸杆菌、异杆菌和阿克曼氏菌等关键细菌,起到改善肥胖糖尿病的症状[66]。在体外发酵试验中,用0.5 mg/mL 桑葚低聚糖培养的鼠李糖酵母菌,可以通过增加拟杆菌和硬壁菌的比率来调节肠道菌群[69]。
桑椹多糖可作为保健食品补充剂的一种功能成分,用于治疗或预防肥胖症。例如,从桑树果实中分离的一种果胶多糖JS-MP-1,通过抑制前脂肪细胞增殖、减少脂肪细胞数量和脂肪组织质量,增加凋亡相关蛋白的表达,剂量依赖性地降低3T3-L1 脂肪细胞的活性[19]。同时,桑葚多糖具有护肝的作用,桑葚多糖可以增加Nrf2 的磷酸化及其核移位,从而激活Nrf2/ARE 信号通路,促进过氧化氢酶和谷胱甘肽过氧化物酶活性[56]。Zhou 等[30]采用急性和亚急性酒精性肝损伤动物模型,评价桑葚多糖的保肝作用。给药后,血清指标和肝脏指标均有所改善。此外,桑葚多糖还能促进内皮细胞一氧化氮的生产,达到降血压的效果[88]。
5 构效关系研究
目前关于桑葚多糖的构效关系研究相对较少,主要集中在初级结构中的分子质量和单糖组成对桑葚多糖的生物活性产生的影响。而桑葚多糖高级结构对其生物活性的影响,目前仍不清楚。
桑葚多糖的生物活性与基本理化性质有关,如单糖组成、分子质量等。桑葚多糖的单糖组成不同,其生物活性也存在较大差异。MFP1、MFP2、MFP3 和MFP4 为桑葚多糖的4 个不同精细组分,MFP 4 由阿拉伯糖、半乳糖和葡萄糖组成,与MFP1、MFP2 和MFP3 单糖组成不同,MFP4 对α-淀粉酶和α-葡萄糖苷酶的抑制作用效果最好[23]。分子质量的不同也会影响桑葚多糖的生理活性,Hu 等[56]采用乙醇分步沉淀法从桑葚中分离出3种多糖组分MFP-1、MFP-2 和MFP-3,其分子质量分别为641.8,115.0 和9.0 ku。分子质量为115.0 ku(MFP-2)的组分能显著减轻棕榈酸引起的肝细胞脂质毒性,而分子质量为641.8 ku(MFP-1)和9.0 ku(MFP-3)的组分保护作用较弱。此外,越来越多的研究发现,对多糖进行化学结构修饰,可以改变其理化性状,提高其生物活性[89-91]。对桑葚多糖采用氧化降解、硫酸化和羧甲基化等方法进行改性后,硫酸化和羧甲基化的桑葚多糖能显著提高乙醇脱氢酶活性。与天然桑葚多糖相比,桑葚多糖衍生物能显著提高乙醇脱氢酶活性,其中引入羧甲基基团比硫酸基团具有更高的护肝效果[60]。可见,多糖的化学修饰引入的官能团对桑葚多糖结构的影响可能改变了其对生物活性。
6 展望
集药、食、补三大功能于一体的桑椹,目前在食品领域中有着广泛的应用,产品种类丰富,如桑葚酸奶[92]、桑葚饮料[93]、桑甚果醋[94]、桑甚果酒[95]等,尤其在酿酒工业及果汁饮品中的应用相对较多。桑葚多糖虽具有抗氧化、降血糖、免疫调节和护肝等药理作用,但目前桑葚多糖的开发还不够充分,主要问题包括:
1)目前桑葚多糖的提取仍以传统的水提醇沉法为主,今后研究方向可以基于果胶型多糖特点优化提取方法,以便更好的进行结构解析。
2)在桑葚多糖的结构仍停留在基础阶段的认识,对其结构深入解析与探讨的报道不多,尤其是对桑葚多糖的构象研究非常缺乏,应深入研究桑葚多糖的不同组分的一级结构。
3)在桑葚多糖的生理活性方面,动物实验和细胞试验虽验证了桑葚多糖的功效,但潜在机制尚不明确,尤其在肠道菌群和代谢方面,应进行更多的动物实验,以获得更多关于桑葚多糖的生物活性的具体机制。
4)虽然关于桑葚多糖的活性有较多报道,但目前还缺乏桑葚多糖的开发与应用,其产品开发比较薄弱。应深入研究桑葚多糖的保健功能,针对不同人群的需求开发桑葚多糖功能食品,实现桑葚多糖产业化。
[1]LI E,YANG S Y,ZOU Y X,et al.Purification,characterization,prebiotic preparations and antioxidant activity of oligosaccharides from mulberries[J].Molecules,2019,24(12):2329.
[2]ZHANG J Q,CHEN C,FU X,et al.A study on the Fe3O4@Fructus mori L.polysaccharide particles with enhanced antioxidant activity and bioavailability[J].Food&Function,2020,11(3):2268-2278.
[3]ERCISLI S,ORHAN E.Chemical composition of white (Morus alba),red (Morus rubra) and black(Morus nigra) mulberry fruits[J].Food Chemistry,2007,103(4):1380-1384.
[4]国家药典委员会.中华人民共和国药典[M].北京:中国医药科技出版社,2015:300.
Chinese Pharmacopoeia Commission.Pharmacopoeia of the People's Republic of China[M].Beijing:China Medical Science Press,2015:300.
[5]陈士铎.本草新编[M].山西:山西科学技术出版社,2011:201.
CHEN S D.New compilation of materia medica[M].Shanxi:Shanxi Science and Technology Publishing House,2011:201.
[6]陈春.桑葚多糖的结构鉴定、活性评价及其体外消化酵解[D].广州:华南理工大学,2018.
CHEN C.Structural identification,biological activities evaluation,digestion and fermentation in vitro of polysaccharides from Fructus Mori[D].Guangzhou:South China University of Technology,2018.
[7]南京中医药大学.中药大辞典:上册[M].第二版.上海:上海科学技术出版社,2006:2790.
Nanjing University of Chinese Medicine.Chinese dictionary of herbal medicine:Volume I[M].2nd ed.Shanghai:Shanghai Scientific&Technical Publishers,2006:2790.
[8]江岩,李斌,郭晓军.新疆黑桑椹多糖的提取和测定[J].食品科学,2008,29(8):224-226.
JIANG Y,LI B,GUO X J.Extraction and determination of polysaccharides from Xinjiang black mulberry[J].Food Science,2008,29(8):224-226.
[9]WEN P,HU T G,LINHARDT R J,et al.Mulberry:A review of bioactive compounds and advanced processing technology[J].Trends in Food Science&Technology,2019,83:138-158.
[10]HE X R,FANG J C,RUAN Y L,et al.Structures,bioactivities and future prospective of polysaccharides from Morus alba (white mulberry):A review[J].Food Chemistry,2018,245:899-910.
[11]KHALIFA I,ZHU W,LI K K,et al.Polyphenols of mulberry fruits as multifaceted compounds:Compositions,metabolism,health benefits,and stability-A structural review [J].Journal of Functional Foods,2018,40:28-43.
[12]TSUDUKI T,KIKUCHI I,KIMURA T,et al.Intake of mulberry 1-deoxynojirimycin prevents dietinduced obesity through increases in adiponectin in mice[J].Food Chemistry,2013,139(1):16-23.
[13]LOU H Q,HU Y,ZHANG L Y,et al.Nondestructive evaluation of the changes of total flavonoid,total phenols,ABTS and DPPH radical scavenging activities,and sugars during mulberry (Morus alba L.) fruits development by chlorophyll fluorescence and RGB intensity values[J].LWT-Food Science and Technology,2012,47(1):19-24.
[14]HSU L S,HO H H,LIN M C,et al.Mulberry water extracts (MWEs) ameliorated carbon tetrachloride-induced liver damages in rat [J].Food and Chemical Toxicology,2012,50(9):3086-3093.
[15]CHANG J J,HSU M J,HUANG H P,et al.Mulberry anthocyanins inhibit oleic acid induced lipid accumulation by reduction of lipogenesis and promotion of hepatic lipid clearance[J].Journal of Agricultural and Food Chemistry,2013,61(25):6069-6076.
[16]CHENG J R,LIU X M,CHEN Z Y,et al.Mulberry anthocyanin biotransformation by intestinal probiotics[J].Food Chemistry,2016,213:721-727.
[17]LOWE J B,MARTH J D.A genetic approach to mammalian glycan function [J].Annual Review of Biochemistry,2003,72(1):643-691.
[18]谢明勇,殷军艺,聂少平.天然产物来源多糖结构解析研究进展[J].中国食品学报,2017,17(3):1-19.
XIE M Y,YIN J Y,NIE S P.Research progress on structural characterization of polysaccharides from natural resources[J].Journal of Chinese Institute of Food Science and Technology,2017,17(3):1-19.
[19]CHOI J W,SYNYTSYA A,CAPEK P,et al.Structural analysis and anti-obesity effect of a pectic polysaccharide isolated from Korean mulberry fruit Oddi (Morus alba L.)[J].Carbohydrate Polymers,2016,146:187-196.
[20]LEE J S,SYNYTSYA A,KIM H B,et al.Purification,characterization and immunomodulating activity of a pectic polysaccharide isolated from Korean mulberry fruit Oddi (Morus alba L.)[J].International Immunopharmacology,2013,17(3):858-866.
[21]LI S J,LI M X,YUE H,et al.Structural elucidation of a pectic polysaccharide from Fructus Mori and its bioactivity on intestinal bacteria strains[J].Carbohydrate Polymers,2018,186:168-175.
[22]WANG W,LI X W,BAO X W,et al.Extraction of polysaccharides from black mulberry fruit and their effect on enhancing antioxidant activity[J].International Journal of Biological Macromolecules,2018,120(Pt B):1420-1429.
[23]CHEN C,YOU L J,ABBASI A M,et al.Characterization of polysaccharide fractions in mulberry fruit and assessment of their antioxidant and hypoglycemic activities in vitro[J].Food&Function,2016,7(1):530-539.
[24]CHEN C,HUANG Q,FU X,et al.In vitro fermentation of mulberry fruit polysaccharides by human fecal inocula and impact on microbiota[J].Food&Function,2016,7(11):4637-4643.
[25]刘金金,殷军艺,黄晓君,等.决明子多糖结构和生物活性功能研究进展[J].食品研究与开发,2019,40(23):212-224.
LIU J J,YIN J Y,HUANG X J,et al.Research progress on structure and biological activity of polysaccharide from Cassia seed[J].Food Research and Development,2019,40(23):212-224.
[26]CHEN W W,JIA Z B,ZHU J J,et al.Optimization of ultrasonic-assisted enzymatic extraction of polysaccharides from thick-shell mussel (Mytilus coruscus) and their antioxidant activities[J].International Journal of Biological Macromolecules,2019,140:1116-1125.
[27]HASHEMIFESHARAKI R,XANTHAKIS E,ALTINTAS Z,et al.Microwave-assisted extraction of polysaccharides from the marshmallow roots:Optimization,purification,structure,and bioactivity[J].Carbohydrate Polymers,2020,240:116301.
[28]ZHU L,LU Y,SUN Z,et al.The application of an aqueous two-phase system combined with ultrasonic cell disruption extraction and HPLC in the simultaneous separation and analysis of solanine and Solanum nigrum polysaccharide from Solanum nigrum unripe fruit[J].Food Chemistry,2020,304:125383.
[29]CHEN G J,FANG C C,CHEN X H,et al.Highpressure ultrasonic-assisted extraction of polysaccharides from Mentha haplocalyx:Structure,functional and biological activities [J].Industrial Crops and Products,2019,130:273-284.
[30]ZHOU X,DENG Q F,CHEN H G,et al.Characterizations and hepatoprotective effect of polysaccharides from Mori Fructus in rats with alcoholic-induced liver injury[J].International Journal of Biological Macromolecules,2017,102:60-67.
[31]CHEN C,ZHANG B,FU X,et al.A novel polysaccharide isolated from mulberry fruits (Murus alba L.) and its selenide derivative:Structural characterization and biological activities [J].Food&Function,2016,7(6):2886-2897.
[32]CHEN C,YOU L J,ABBASI A M,et al.Optimization for ultrasound extraction of polysaccharides from mulberry fruits with antioxidant and hyper glycemic activity in vitro[J].Carbohydrate Polymers,2015,130:122-132.
[33]WEI W,ZHOU W,ZANG N,et al.Structural analysis of a polysaccharide from Fructus Mori Albae[J].Carbohydrate Polymers,2007,70(3):341-344.
[34]冯斌,朱保昆,廖头根,等.高效提取桑葚多糖的工艺优化研究[J].食品工业,2014,35(4):8-11.
FENG B,ZHU B K,LIAO T G,et al.Efficient extraction and process optimization of Fructus Mori polysaccharide by HPEF[J].The Food Industry,2014,35(4):8-11.
[35]王强,王睿,王存,等.桑葚多糖调节血糖代谢及体外抗氧化效果研究[J].食品科学,2014,35(11):260-264.
WANG Q,WANG R,WANG C,et al.Effects of mulberry polysaccharides on glucose metabolism and their antioxidant activities in vitro[J].Food Science,2014,35(11):260-264.
[36]景荣琴,熊清平,景怡.响应面法优化桑葚多糖的超声波辅助提取工艺条件[J].天然产物研究与开发,2014,26(4):570-574.
JING R Q,XIONG Q P,JING Y.Optimization of ultrasonic-assisted extraction of polysaccharides from Fructus Mori by response surface methodology [J].Natural Product Research and Development,2014,26(4):570-574.
[37]杨桦,邝思碧,邹宇晓,等.酶法协同微波提取桑椹多糖工艺优化的研究[J].食品研究与开发,2018,39(10):32-37.
YANG H,KUANG S B,ZOU Y X,et al.Optimization of enzyme-microwave assisted extraction process of polysaccharide from mulberry[J].Food Research and Development,2018,39(10):32-37.
[38]魏兆军,胡海梅,柏晓辉,等.桑椹多糖提取工艺的优化[J].食品科学,2007,28(11):261-264.
WEI Z J,HU H M,BO X H,et al.Optimization of extraction of polysaccharide from mulberry fruit[J].Food Science,2007,28(11):261-264.
[39]DENG Q F,ZHOU X,CHEN H G.Optimization of enzyme assisted extraction of Fructus Mori polysaccharides and its activities on antioxidant and alcohol dehydrogenase [J].Carbohydrate Polymers,2014,111:775-782.
[40]WANG D X,LI H,LI B,et al.Systematic fractionation and immunoenhancement of water-soluble polysaccharides isolated from fruit of Morus alba L.[J].International Journal of Biological Macromolecules,2018,116:1056-1063.
[41]TANG W,LIU D,YIN J Y,et al.Consecutive and progressive purification of food-derived natural polysaccharide:Based on material,extraction process and crude polysaccharide[J].Trends in Food Science&Technology,2020,99:76-87.
[42]COLODEL C,VRIESMANN L C,DE OLIVEIRA PETKOWICZ C L.Cell wall polysaccharides from Ponkan mandarin (Citrus reticulata Blanco cv.Ponkan) peel [J].Carbohydrate Polymers,2018,195:120-127.
[43]CHEN X,ZHANG H B,DU W Q,et al.Comparison of different extraction methods for polysaccharides from Crataegus pinnatifida Bunge[J].International Journal of Biological Macromolecules,2020,150:1011-1019.
[44]HE L A,YAN X T,LIANG J,et al.Comparison of different extraction methods for polysaccharides from Dendrobium officinale stem [J].Carbohydrate Polymers,2018,198:101-108.
[45]MARIC M,GRASSINO A N,ZHU Z,et al.An overview of the traditional and innovative approaches for pectin extraction from plant food wastes and byproducts:Ultrasound-,microwaves-,and enzymeassisted extraction[J].Trends in Food Science&Technology,2018,76:28-37.
[46]DRANCA F,OROIAN M.Extraction,purification and characterization of pectin from alternative sources with potential technological applications[J].Food Research International,2018,113:327-350.
[47]谢明勇,聂少平.天然产物活性多糖结构与功能研究进展[J].中国食品学报,2010,10(2):1-11.
XIE M Y,NIE S P.A review about the research of structure and function of polysaccharides from natural products[J].Journal of Chinese Institute of Food Science and Technology,2010,10(2):1-11.
[48]CHEN L,HUANG G L,HU J C.Preparation,deproteinization,characterisation,and antioxidant activity of polysaccharide from cucumber (Cucumis saticus L.) [J].International Journal of Biological Macromolecules,2018,108:408-411.
[49]ZHAO Q S,XIE B X,YAN J,et al.In vitro antioxidant and antitumor activities of polysaccharides extracted from Asparagus officinalis[J].Carbohydrate Polymers,2012,87(1):392-396.
[50]ZENG X T,LI P Y,CHEN X,et al.Effects of deproteinization methods on primary structure and antioxidant activity of Ganoderma lucidum polysaccharides[J].International Journal of Biological Macromolecules,2019,126:867-876.
[51]刘玉佳,孔繁东,刘兆芳,等.桔梗多糖Sevag 法除蛋白工艺的研究[J].中国调味品,2014,39(4):5-7.
LIU Y J,KONG F D,LIU Z F,et al.Research on technology of deproteinization from polysaccharide of Platycodon grandiflorum by Sevag method[J].China Condiment,2014,39(4):5-7.
[52]孙军涛.玉米芯低聚木糖脱色工艺研究[J].食品工业,2016,37(7):108-111.SUN J T.Study on the decolorization process of xylooligosaccharides from corncobs[J].The Food Industry,2016,37(7):108-111.
[53]曹鹏伟,戴军,彭奇均.树脂对灵芝多糖色素吸附研究[J].食品工业科技,2009,30(10):90-93.
CAO P W,DAI J,PENG Q J.Study on adsorption for Ganoderma lucidum polysaccharide pigment with resin[J].Science and Technology of Food Industry,2009,30(10):90-93.
[54]杨大伟,吴永尧,唐巧玉.碎米荠多糖的过氧化氢脱色方法研究[J].食品科技,2008,29(1):174-177,181.
YANG D W,WU Y Y,TANG Q Y.Study on methods for decolorizing polysaccharide from Cardamine enshiensis by hydrogen peroxide[J].Food Science and Technology,2008,29(1):174-177,181.
[55]尹艳,高文宏,于淑娟.多糖提取技术的研究进展[J].食品工业科技,2007,28(2):248-250.
YIN Y,GAO W H,YU S J.Research progress of polysaccharide extraction technology[J].Science and Technology of Food Industry,2007,28(2):248-250.
[56]HU D W,BAO T,LU Y,et al.Polysaccharide from mulberry fruit (Morus alba L.) protects against palmitic-acid-induced hepatocyte lipotoxicity by activating the Nrf2/ARE signaling pathway[J].Journal of Agricultural and Food Chemistry,2020,68(46):13016-13024.
[57]WANG P P,HUANG Q,CHEN C,et al.The chemical structure and biological activities of a novel polysaccharide obtained from Fructus Mori and its zinc derivative[J].Journal of Functional Foods,2019,54:64-73.
[58]CHEN C,WANG P P,HUANG Q,et al.A comparison study on polysaccharides extracted from Fructus Mori using different methods:Structural characterization and glucose entrapment[J].Food Function,2019,10(6):3684-3695.
[59]张培丽,张帅,陈雪香,等.桑葚多糖对H2O2 诱导PC-12 细胞氧化损伤的保护作用[J].现代食品科技,2015,31(11):20-24,85.
ZHANG P L,ZHANG S,CHEN X X,et al.Protective effect of mulberry polysaccharides on H2O2-induced oxidative damage in PC-12 cells[J].Modern Food Science and Technology,2015,31(11):20-24,85.
[60]DENG Q F,WANG X,CHEN H G,et al.Structural characterization,modification and hepatoprotective effects of polysaccharide from Mori Fructus[J].International Journal of Biological Macromolecules,2020,153:357-363.
[61]ZHANG J,CHEN C,FU X.Fructus mori L.polysaccharide-iron chelates formed by self-embedding with iron(iii) as the core exhibit good antioxidant activity[J].Food&Function,2019,10(6):3150-3160.
[62]CHEN C,HUANG Q,YOU L J,et al.Chemical property and impacts of different polysaccharide fractions from Fructus Mori.on lipolysis with digestion model in vitro[J].Carbohydrate Polymers,2017,178:360-367.
[63]王杏,邓青芳,陈华国,等.桑椹多糖的结构表征及其对乙醇脱氢酶活性的影响研究[J].中国中药杂志,2017,42(12):2329-2333.
WANG X,DENG Q F,CHEN H G,et al.Characterization and activity effect on ADH of polysaccharides from Mori fructus[J].China Journal of Chinese Materia Medica,2017,42(12):2329-2333.
[64]易建勇,毕金峰,刘璇,等.果胶结构域精细结构研究进展[J].食品科学,2020,41(7):292-299.
YI J Y,BI J F,LIU X,et al.A review:Domain fine structure of pectic polysaccharides[J].Food Science,2020,41(7):292-299.
[65]VORAGEN A G J,COENEN G J,VERHOEF R P,et al.Pectin,a versatile polysaccharide present in plant cell walls[J].Structural Chemistry,2009,20(2):263-275.
[66]CHEN C,YOU L J,HUANG Q,et al.Modulation of gut microbiota by mulberry fruit polysaccharide treatment of obese diabetic db/db mice[J].Food&Function,2018,9(7):3732-3742.
[67]SHIN B R,KIM H S,YUN M J,et al.Promoting effect of polysaccharide isolated from Mori fructus on dendritic cell maturation[J].Food and Chemical Toxicology,2013,51:411-418.
[68]LIU C J,LIN J Y.Anti-inflammatory and antiapoptotic effects of strawberry and mulberry fruit polysaccharides on lipopolysaccharide-stimulated macrophages through modulating pro-/anti-inflammatory cytokines secretion and Bcl-2/Bak protein ratio[J].Food and Chemical Toxicology,2012,50(9):3032-3039.
[69]LI E,YANG H,ZOU Y X,et al.In-vitro digestion by simulated gastrointestinal juices of Lactobacillus rhamnosus cultured with mulberry oligosaccharides and subsequent fermentation with human fecal inocula[J].LWT-Food Science and Technology,2019,101:61-68.
[70]JIAO Y K,WANG X Q,JIANG X,et al.Antidiabetic effects of Morus alba fruit polysaccharides on high-fat diet-and streptozotocin-induced type 2 diabetes in rats [J].Journal of Ethnopharmacology,2017,199:119-127.
[71]DATTA H K,DAS D,KOSCHELLA A,et al.Structural elucidation of a heteropolysaccharide from the wild mushroom Marasmiellus palmivorus and its immune-assisted anticancer activity[J].Carbohydrate Polymers,2019,211:272-280.
[72]JO M,JUNG J H,KIM H W,et al.Polysaccharide isolated from fermented barley activates innate immune system and anti-tumor metastasis in mice[J].Journal of Cereal Science,2020,92:102919.
[73]PRAVEEN M A,PARVATHY K R K,BALASUBRAMANIAN P,et al.An overview of extraction and purification techniques of seaweed dietary fibers for immunomodulation on gut microbiota[J].Trends in Food Science&Technology,2019,92:46-64.
[74]ZHAO Y,YAN Y M,ZHOU W T,et al.Effects of polysaccharides from bee collected pollen of Chinese wolfberry on immune response and gut microbiota composition in cyclophosphamide-treated mice[J].Journal of Functional Foods,2020,72:104057.
[75]FERREIRA S S,PASSOS C P,MADUREIRA P,et al.Structure-function relationships of immunostimulatory polysaccharides:A review[J].Carbohydrate Polymers,2015,132:378-396.
[76]RAMBERG J E,NELSON E D,SINNOTT R A.Immunomodulatory dietary polysaccharides:A systematic review of the literature[J].Nutrition Journal,2010,9:54.
[77]GUILLIAMS M,GINHOUX F,JAKUBZICK C,et al.Dendritic cells,monocytes and macrophages:A unified nomenclature based on ontogeny[J].Nature Reviews Immunology,2014,14(8):571-578.
[78]BENNACEUR K,CHAPMAN J A,TOURAINE J L,et al.RETRACTED:Immunosuppressive networks in the tumour environment and their effect in dendritic cells [J].Biochimica et Biophysica Acta(BBA)-Reviews on Cancer,2009,1795(1):16-24.
[79]MILLER G,LAHRS S,DEMATTEO R P.Overexpression of interleukin-12 enables dendritic cells to activate NK cells and confer systemic antitumor immunity[J].FASEB Journal,2003,17(6):728-730.
[80]FEARON D T,LOCKSLEY R M.The Instructive role of innate immunity in the acquired immune response[J].Science,1996,272(5258):50-54.
[81]CHEN C,HUANG Q,LI C,et al.Hypoglycemic effects of a Fructus Mori polysaccharide in vitro and in vivo[J].Food&Function,2017,8(7):2523-2535.
[82]WITHERS D J,BURKS D J,TOWERY H H,et al.Irs-2 coordinates Igf-1 receptor-mediated betacell development and peripheral insulin signalling[J].Nature Genetics,1999,23(1):32-40.
[83]WITHERS D J,GUTIERREZ J S,TOWERY H,et al.Disruption of IRS-2 causes type 2 diabetes in mice[J].Nature,1998,391(6670):900-904.
[84]CHEN Z Y,DU X,YANG Y Y,et al.Comparative study of chemical composition and active components against α-glucosidase of various medicinal parts of Morus alba L.[J].Biomedical chromatography:BMC,2018,32(11):e4328.
[85]CHEN G J,LI C F,WANG S S,et al.Characterization of physicochemical properties and antioxidant activity of polysaccharides from shoot residues of bamboo (Chimonobambusa quadrangularis):Effect of drying procedures [J].Food Chemistry,2019,292:281-293.
[86]LI J E,NIE S P,XIE M Y,et al.Isolation and partial characterization of a neutral polysaccharide from Mosla chinensis Maxim.cv.Jiangxiangru and its antioxidant and immunomodulatory activities [J].Journal of Functional Foods,2014,6:410-418.
[87]LEE J,MA K,MOULIK M,et al.Untimely oxidative stress in β-cells leads to diabetes-Role of circadian clock in β-cell function[J].Free Radical Biology and Medicine,2018,119:69-74.
[88]WANG C Y,CHENG W H,BAI S W,et al.White mulberry fruit polysaccharides enhance endothelial nitric oxide production to relax arteries in vitro and reduce blood pressure in vivo[J].Biomedicine&Pharmacotherapy,2019,116:109022.
[89]HUANG L X,HUANG M,SHEN M Y,et al.Sulfated modification enhanced the antioxidant activity of Mesona chinensis Benth polysaccharide and its protective effect on cellular oxidative stress[J].International Journal of Biological Macromolecules,2019,136:1000-1006.
[90]LI Y,YUAN Y,LEI L,et al.Carboxymethylation of polysaccharide from Morchella angusticepes Peck enhances its cholesterol-lowering activity in rats[J].Carbohydrate Polymers,2017,172:85-92.
[91]KLEIN-KOERKAMP C,GRANET R,ZERROUKI R,et al.Efficient oxidative modification of polysaccharides in water using H2O2 activated by iron sulfophthalocyanine[J].Carbohydrate Polymers,2009,78(4):938-944.
[92]程水明,曾霞,周国钰,等.桑葚复合酸奶的研制[J].中国酿造,2015,34(10):147-150.
CHENG S M,ZENG X,ZHOU G Y,et al.Development of mulberry compound yoghurt[J].China Brewing,2015,34(10):147-150.
[93]TOMAS M,TOYDEMIR G,BOYACIOGLU D,et al.The effects of juice processing on black mulberry antioxidants[J].Food Chemistry,2015,186:277-284.
[94]汪晓琳.双菌株混合发酵生产桑葚果醋工艺的研究[J].中国调味品,2017,42(7):100-105.
WANG X L.Research on technology of mulberry fruit vinegar fermented with double strains[J].China Condiment,2017,42(7):100-105.
[95]ZHANG L X,FAN G J,KHAN M A,et al.Ultrasonic-assisted enzymatic extraction and identification of anthocyanin components from mulberry wine residues[J].Food Chemistry,2020,323:126714.