不同强度的运动对机体的免疫功能影响不同,中、小强度运动可以提高机体免疫功能,降低感染疾病的风险,而剧烈运动或过度负荷训练则会暂时抑制免疫功能,导致运动性免疫抑制发生,主要表现为上呼吸道和消化道症状的风险升高[1-2]。耐力运动员由于训练和比赛期间的强度都较大,因此更容易出现上呼吸道感染(Upper respiratory tract infection,URTI)和胃肠道不适[3-4]。寻找有效的干预手段提升运动员机体免疫功能十分重要。
肠道内含有多种微生物,统称为肠道菌群。正常情况下,人体肠道菌群的种类和数量达到平衡状态,以专性厌氧菌如双歧杆菌和乳酸杆菌等为优势菌,能够抑制有害菌和病原体等对肠道的破坏,维持人体健康[5]。此外,微生物的代谢产物短链脂肪酸(Short chain fatty acids,SCFA)也具有维持肠道黏膜屏障、调节免疫、抗炎以及为机体提供能量等作用[6]。研究发现,适度运动可以改变肠道菌群的组成和多样性,增加促进健康的细菌丰度以及SCFA 含量,从而增强机体的适应性[7]。然而,剧烈运动和应激可打破肠道菌群的平衡,引起致病共生微生物群的数量增加,优势有益物种的数量减少[8]。如何在剧烈运动时维持肠道菌群的稳态十分关键。益生菌是活的微生物,在使用足够量时对健康有益,如改善肠道菌群组成,促进有益菌生长;产生抗菌物质来抑制或杀死致病菌;促进胃肠蠕动,改善机体的消化与吸收;增强体液免疫应答,提升机体免疫[9]。尽管其作用机制尚未明确,但一些研究表明,定期补充益生菌可积极改变肠道菌群的数量和结构,增强体液免疫和肠黏膜防御功能,进而减轻URTI 和胃肠道症状的发生率及其症状严重程度[10]。基于此,近年来有多种益生菌作为重要的营养补剂应用于运动领域,并通过减轻疾病对机体的负面影响而对运动表现有帮助[11]。最常应用的菌种有干酪乳杆菌、发酵乳杆菌、双歧杆菌、植物乳杆菌、嗜酸乳杆菌和鼠李糖乳杆菌等[12]。目前的研究主要评估了益生菌在减轻运动引起的免疫功能抑制、预防或缓解运动期间上呼吸道或胃肠道症状的效果,然而,因使用菌种的差异,不同研究报道益生菌的效果不一致,相关的人体研究也较少。本文综述运动性免疫抑制的原因,对运动员运动性免疫抑制有益的益生菌,益生菌干预措施和干预结果的差异,探讨益生菌提高运动员免疫机能的作用及机制,旨在为益生菌应用于运动领域提供理论依据和参考。
1 运动性免疫抑制及其原因
1.1 大强度运动后免疫细胞功能下降
运动性免疫抑制与大强度运动后机体免疫细胞功能下降有关。有报道指出,大强度运动可导致自然杀伤细胞[13]和中性粒细胞活性降低,1 型T 淋巴细胞数目显著减少,CD4+/CD8+比值下降[14-17],B淋巴细胞和T 淋巴细胞的增殖能力以及巨噬细胞的吞噬能力也下降[18]。此外,剧烈运动引起的皮质醇升高也可抑制单核细胞/巨噬细胞抗原呈递和T淋巴细胞的功能[19]。大强度运动后机体免疫细胞数量的减少及功能的下降可能会导致所谓的“开窗期”,在此期间运动员出现感染的几率升高。
1.2 大强度运动后免疫因子的变化
大强度运动会对免疫产生负面影响,并改变炎症反应,包括促炎/抗炎细胞因子[19]。过度训练引起的组织损伤可导致细胞因子,如肿瘤坏死因子α(Tumour necrosis factor alpha,TNF-α)、白细胞介素-1(IL-1)、白细胞介素-8(IL-8)和白细胞介素-6(IL-6)的过度生成,使运动员出现慢性疲劳,诱发体液反应,从而抑制了细胞介导的免疫反应,导致出现感染几率更高[20]。大强度运动还可降低干扰素γ (Interferon gamma,IFN-γ) 水平,而IFN-γ 可以通过调节辅助型T 细胞1(T helper 1 cell,Th1) 和辅助型T 细胞2 (T helper 2 cell,Th2) 亚群数量使其比值达到平衡状态来缓解炎症[21]。除此之外,大强度运动导致的免疫抑制与免疫球蛋白分泌减少有关,尤其是唾液免疫球蛋白A(Immunoglobulins A,IgA)和免疫球蛋白M(Immunoglobulins M,IgM)。IgA 由黏膜表面的B 细胞分泌,是最丰富的非炎性免疫球蛋白[22],当其分泌到黏膜后形成分泌型IgA (Secretory immunoglobulin A,SIgA)。SIgA 存在于黏膜表面和黏膜分泌液(如唾液和消化液)中,是黏膜免疫的第一道防线,也是反映机体黏膜免疫系统状态的指标。它与覆盖在上皮细胞的黏蛋白结合,可抵抗外源入侵的病原体。研究报道,唾液SIgA 在机体抵御URTI 过程中具有重要的作用,剧烈运动和长时间大强度训练会抑制唾液SIgA 的分泌[23]。例如,马拉松比赛后以及精英男足球员进行足球比赛后,唾液SIgA 的水平分别降低了约50%[24]和75%[25],并且训练与比赛负荷的增加可导致唾液SIgA 水平显著下降,这与耐力运动员URTI 风险升高密切相关[23,26]。
1.3 大强度运动影响肠黏膜免疫稳态
肠黏膜不仅是机体消化和吸收营养物质的重要场所,也是抵御细菌、病原体等外源性物质侵入机体的第一道防线[27]。肠黏膜上有丰富的淋巴组织,它们通过细胞免疫和体液免疫共同抵御细菌和病原体等的侵害。大强度运动或过度训练会引起消化道灌注不足,破坏黏膜屏障完整性,导致肠道通透性升高,继而诱发炎症反应,增加肠道相关疾病的患病风险[28]。30%~50%的运动员,尤其是耐力项目运动员,会出现胃肠道不适症状,包括上腹部不适、肠胃气胀、恶心和呕吐等[29-30]。肠黏膜屏障主要由上皮细胞间的紧密连接蛋白(包括咬合蛋白Occludin、闭合蛋白Claudins 和闭合小环蛋白ZO-1)和覆盖在肠上皮细胞的黏液层构成,共同抵御肠内的有害物质进入肠组织及血液循环。其受损后可增加细菌移位和内毒素转运,如脂多糖(Lipopolysaccharides,LPS),甚至导致内毒素血症[30]。研究发现,大强度负重游泳可导致小肠黏膜形态发生改变,黏膜上皮细胞之间的间隙变宽,并且肠组织咬合蛋白Occludin 和闭合蛋白Claudins的表达降低,肠黏膜屏障功能受损[31-32]。此外,大强度运动还促进促炎细胞因子分泌。跑台力竭运动可导致小鼠小肠通透性增加,小肠组织TNF-α 和IL-2 的表达也显著升高[33]。研究证实,耐力运动员力竭运动或比赛后血浆LPS 浓度显著升高,并且LPS 水平与出现胃肠道症状的风险呈正相关[34-35]。
2 益生菌减轻运动引起的免疫功能抑制
2.1 补充益生菌降低上呼吸道感染发生率及症状程度
长时间运动和大负荷训练可能导致URTI,出现如咳嗽、打喷嚏、充血和喉咙痛等症状,多见于耐力运动员,而反复出现的上呼吸道症状可能会影响运动表现。研究表明某些益生菌可减少URTI的发病率,并降低其症状的严重程度[36]。然而,由于不同的研究选取的益生菌,以及试验方案和结果指标的差异使得结论变得复杂。
几项研究强调了益生菌可降低耐力运动员URTI 的发病率和症状严重程度。Cox 等[37]和West等[38]分别给长跑和自行车运动员补充发酵乳杆菌VRI-003(PCC),Gleeson 等[39]给耐力运动员补充干酪乳杆菌Shirota 的研究都得出了明确的结论。也有文献报道,优秀运动员在冬季训练期间补充瑞士乳酸杆菌Lafti L10 虽然并不改善呼吸道疾病的严重程度和发病率,但可减少呼吸道感染症状的持续时间[40]。此外,多种益生菌联合补充也有相似效果。Strasser 等[41]报道在冬季训练3 个月期间补充多种益生菌可显著降低运动员进行疲劳有氧运动后URTI 的发病率。高水平橄榄球运动员补充多种乳杆菌(4 周)也可降低URTI 的发病率,缩短患病天数,但不影响呼吸道症状的严重程度[42]。有研究表明,短期补充益生菌也有积极效果。乳球菌服用14 d 可降低男性大学生运动员URTI 的发病率,并缓解打喷嚏或流鼻涕等症状[43]。West 等[44]针对健康个体的研究发现动物双歧杆菌也可降低URTI 的风险,提示通常被认为处于URTI 低风险的健康活跃人群也可以从益生菌补充剂中受益。以上研究提示,益生菌可作为一种营养补充剂用于减少运动员URTI 的发生率、持续时间和严重程度。然而,也有学者报道了不一致的结果。Kekkonen 等[45]给141 名马拉松运动员服用鼠李糖乳杆菌3 个月,发现在马拉松比赛后的两周内益生菌组URTI 的发病率并未下降。Gleeson 等[46]研究了益生菌对243 名大学耐力运动员URTI 发生率的影响,发现补充干酪乳杆菌Shirota(持续20周)并未产生积极作用,这可能与受试者本身较低的URTI 发病率有关。由于目前关于益生菌降低运动员URTI 发病率的报道大多局限于乳杆菌属,机制尚不十分明确,因此需要更大规模的试验研究将URTI 发生率和症状程度与免疫反应的其它标志物结合起来,更全面地了解不同的益生菌如何影响免疫系统。
2.2 益生菌改善运动员黏膜免疫功能的机制
益生菌对呼吸系统疾病改善作用主要是通过刺激/调节免疫系统来实现的。在肠道内,益生菌可直接或间接调节免疫细胞功能,进而调节全身免疫,起到预防和缓解疾病的作用。益生菌已被证明可以通过上调吞噬细胞和自然杀伤细胞活性来增强先天免疫(第一道防线),并通过改善抗原呈递、T 淋巴细胞和B 淋巴细胞的功能来增强获得性免疫[47]。嗜酸乳杆菌和长双歧杆菌补充2 周还可减轻运动员一次力竭运动导致的血清淋巴细胞和单核细胞数目减少[48]。补充益生菌还可降低炎症因子的分泌,诱导抗炎反应。一项研究给男性马拉松运动员在马拉松比赛前30 d 补充含有干酪乳杆菌Shirota(4×1010 CFU)的发酵乳,发现益生菌干预可改善全身和气道的免疫反应,包括减少鼻黏膜中性粒细胞浸润、降低上呼吸道中促炎细胞因子水平 (如IL-1、IL-5、IL-6、IL-13 和TNF-α)和升高抗炎细胞因子(IL-10)水平[49]。橄榄球运动员在比赛期间补充大剂量多菌株益生菌27 周后,唾液α-淀粉酶(黏膜免疫反应的标志)显著增加[50]。以上结果表明,每天摄入益生菌能够诱导抗炎反应,从而减轻大强度运动对黏膜炎症的有害作用。
研究证实,调节T 细胞功能是补充益生菌提高机体免疫机能作用的重要环节。通过与树突状细胞的相互作用,益生菌促进部分T 细胞分化为Th1、Th2 和调节性T 细胞 (Regulatory cells,Treg)细胞,激活免疫系统,提高机体免疫[51]。据报道,瑞士乳酸杆菌Lafti L10 可升高冬季训练期间优秀运动员CD4+与CD8+的比值,此比值的降低被认为与急性病毒性感染相关[40]。动物双歧杆菌和嗜酸乳杆菌也可减少马拉松运动员比赛后淋巴细胞产生的促炎细胞因子[17]。Clancy 等[14]研究发现,优秀运动员疲劳运动后T 细胞免疫被抑制,血液中由CD4+的T 细胞分泌的IFN-γ 含量明显降低,而补充嗜酸乳杆菌4 周后,疲劳运动员T 细胞产生的IFN-γ 增加,非疲劳运动员的唾液中IFN-γ浓度也升高。此研究首次证明嗜酸乳杆菌具有逆转T 细胞缺陷,增强黏膜IFN-γ 浓度的能力,并且其作用可能与机体本身的免疫状态有关。之后,Cox 等[37]报道优秀男性长跑运动员在4 个月冬训期间补充发酵乳杆菌VRI-003 可显著升高全血的IFN-γ 含量。鉴于T 细胞在免疫稳态中发挥的核心作用,补充益生菌调节T 细胞的功能的机制值得深入研究。
益生菌还可促进B 淋巴细胞向浆细胞分化,增加IgA 的分泌[52]。几项研究证实了干酪乳杆菌Shirota 维持运动员训练或比赛后唾液IgA 的水平的能力。Gleeson 等[39]研究表明,在4 个月冬训期间给耐力运动员补充含有干酪乳杆菌Shirota 的发酵乳可显著升高唾液IgA 的浓度,伴随着URTI发病率降低。最近,Vaisberg 等[49]也观察到在马拉松比赛前30 d 补充相同益生菌可维持马拉松比赛后唾液IgA 的水平。然而,Gill 等[53]发现高剂量干酪乳杆菌补充7 d 并不能提高耐力运动员劳累热应激后唾液中的抗菌蛋白和SIgA 水平,这可能与益生菌补充时间过短有关。此外,补充其它益生菌的几项研究并未发现唾液IgA 有明显变化,如强化训练阶段的女性游泳运动员补充6 周的双歧杆菌[54]、大学棒球运动员补充枯草芽孢杆菌DE111[55]以及疲劳的运动员补充嗜酸乳杆菌均不影响唾液IgA 水平[14]。目前,关于益生菌维持或升高唾液IgA 水平的研究主要集中在干酪乳杆菌,其它益生菌是否有此作用仍需明确。
虽然并非所有的研究都证明益生菌对机体免疫力的提升作用[56],但总体而言,补充益生菌对运动员免疫系统的影响是积极和有益的,可用于预防过度劳累相关的感染性疾病。然而,目前关于益生菌对易患感染性疾病运动员血液中免疫相关指标影响的研究较少,其作用机制仍不清楚。
3 益生菌改善肠道免疫
3.1 益生菌缓解胃肠症状和肠黏膜屏障通透性
虽然不能消除胃肠道的低灌注,但补充益生菌已被证明是预防和治疗运动导致的胃肠道症状较为有效且安全的方法[57]。乳酸杆菌、双歧杆菌和鼠李糖乳杆菌等特定菌属可以改善一次高强度运动或耐力运动引起的胃肠道不适。研究发现,休闲跑步者服用多株益生菌(嗜酸乳杆菌、双歧杆菌和动物双歧杆菌)28 d 可显著降低马拉松比赛中胃肠道症状严重程度,并且在比赛最后三分之一期间跑步速度的降低与主观胃肠道症状的严重程度之间存在显著相关性[29]。补充鼠李糖乳杆菌3 个月可显著缩短马拉松运动员赛后胃肠道症状发作的持续时间[45]。此外,精英橄榄球运动员补充益生菌4 周后,可显著降低胃肠道疾病发生率[42]。也有研究表明,补充益生菌可减轻运动引起的肠道通透性增加,维持肠黏膜屏障完整性。Lamprecht等[58]发现补充双歧杆菌三联活菌(长型双歧杆菌、嗜酸乳杆菌和粪肠球菌) 可降低男性运动员一次大强度运动后粪便中连蛋白(肠黏膜屏障的标志物之一)的含量。服用多菌株益生菌也可显著改善高温环境中男性跑步者一次力竭运动后[34]以及铁人三项运动员赛后[35]血清LPS 水平,并减少胃肠道的不适症状。并且,Shing 等[34]还发现男性跑步者在高温环境中运行至疲劳的时间增加,这可能与肠道通透性降低有关。虽然胃肠道通透性和屏障功能并不总是与运动相关的胃肠道症状有关,但这并不影响缓解肠黏膜屏障损伤的重要性以及益生菌的重要治疗作用。然而也有一些研究表明,补充益生菌对维持胃肠道健康没有任何帮助。耐力运动员短时间、高剂量的补充干酪乳杆菌后,劳累热应激诱导的肠道通透性增加以及唾液抗菌蛋白水平下降并未得到改善[59],这可能是益生菌补充时间过短,尚未在肠道定殖导致的。总的来讲,补充益生菌是缓解运动员在训练期间出现胃肠道问题的一种较为有效的策略。
3.2 益生菌改善肠黏膜免疫屏障功能的机制
3.2.1 益生菌调节肠道菌群,增强肠黏膜屏障益生菌可通过调节肠道微生物群组成、上调紧密连接蛋白的表达、促进黏液合成来改善肠道防御[60-62](图1)。自行车运动员补充发酵乳杆菌11周后,粪便中乳杆菌数量增加了7.7 倍[38],并且益生菌在肠道的定殖数量与胃肠道症状的减轻程度成正比[34]。动物双歧杆菌发酵乳可减轻大鼠一次高强度运动诱导的肠道菌群变化以及肠黏膜屏障的损伤[63]。在健康成年人中预防性服用唾液乳杆菌UCC118 也可减轻运动引起的肠道通透性升高,并重塑肠道微生物组,表现为疣微菌门(Verrucomicrobia)丰度显著降低,产生丁酸的玫瑰果属(Roseburia)和鞭毛藻科(Lachnospiraceae)丰度显著升高[64]。丁酸是微生物的代谢产物SCFA 的一种,可通过升高紧密连接蛋白表达从而增强肠上皮细胞间的屏障[6]。也有研究发现,肠腔的乳酸菌会产生乳酸,而乳酸可被丁酸产生菌转化为丁酸[65]。因此,益生菌也可能通过增加乳酸菌的定殖升高丁酸水平来发挥保护作用。除此之外,植物乳杆菌、干酪乳杆菌、短乳杆菌和婴儿双歧杆菌可通过上调咬合蛋白Occludin、闭合小环蛋白ZO-1 和闭合蛋白Claudins 的表达减轻黏膜屏障损伤和炎症程度[66-68]。通过调节核因子κB(Nuclear factor kappa beta,NF-κB)、丝裂原活化蛋白激酶(MAPK)和蛋白激酶C(PKC)信号通路,益生菌可减少上皮细胞凋亡,阻止紧密连接蛋白发生变性[51]。鼠李糖乳杆菌、双歧杆菌和乳酸杆菌也能促进黏液合成和分泌,增强黏液屏障,从而减少致病菌的黏附[69-71]。
3.2.2 益生菌改善肠道免疫防御 抑制炎症因子表达、促进SIgA 分泌是益生菌改善肠道免疫功能的重要途径(图1)。益生菌通过调节关键信号通路来调节肠上皮细胞、巨噬细胞和树突状细胞的细胞因子分泌,如NF-κB、MAPK、PKC 与转录激活因子(Signal transducers and activators of transcription,STAT)等通路[51]。研究发现,嗜酸乳杆菌和双歧杆菌等益生菌通过减少炎症因子TNF-α、IL-6、白细胞介素1β(IL-1β)和IL-8 的表达以及中性粒细胞浸润来缓解肠黏膜炎症损伤[69-70],并降低肠道通透性[71]。除此之外,益生菌还可促进肠道浆细胞分泌IgA,防止病原体在肠道内繁殖,从而增强肠道免疫屏障。Kabeerdoss 等[72]给年轻健康女性补充含有乳酸双歧杆菌Bb12 的酸奶3 周,发现在益生菌补充期间粪便IgA 水平显著升高。含有干酪乳杆菌的酸奶也可增加小鼠肠液中的SIgA水平[73]。综上所述,益生菌通过调节肠道菌群、增强肠黏膜屏障功能和抑制炎症因子表达来增强肠黏膜免疫功能。
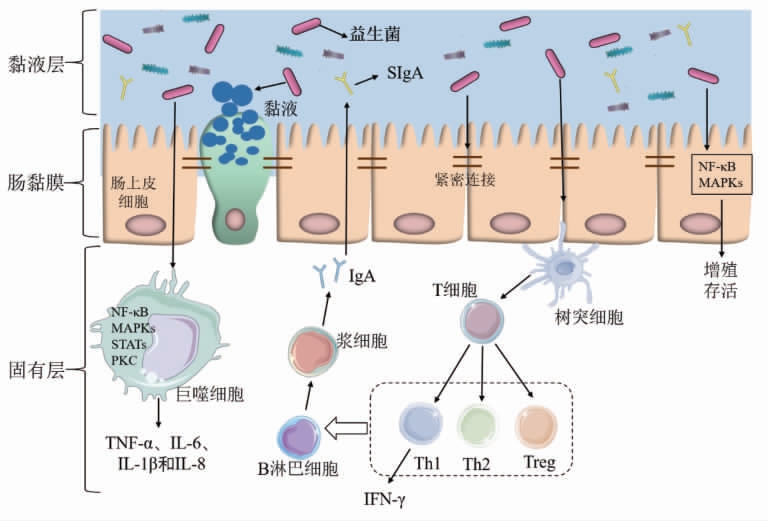
图1 益生菌对黏膜免疫影响示意图
Fig.1 Schematic of probiotics effects on mucosal immune function
4 总结与展望
剧烈运动会使运动员陷入困境,产生运动性免疫抑制,更容易受到感染和炎症的影响。益生菌可调节宿主的免疫和炎症反应,增强黏膜免疫,降低URTI 的发病率、严重程度和/或持续时间;还可以优化肠道菌群构成,增强肠黏膜屏障功能,减轻运动员胃肠道不适的发病率及持续时间,从而对增强运动员免疫功能具有良好影响 [10](图1)。然而,服用益生菌的效果受到益生菌干预的剂量和时间、配方以及菌株定殖能力的影响。目前,益生菌以胶囊、粉剂或添加到某些乳制品(发酵乳或酸奶) 的形式补充,剂量通常在1×109~4×1010 CFU范围内[47,74]。在一些短期研究中(通常小于4 周),益生菌发挥生理效应的时间可能太短,无法真实地评估其长期意义。现有研究中益生菌的干预时长 为4~21 周[47,74]。Haywood 等[42]、Strasser 等[41]和Roberts 等[35]的研究显示,在大多数情况下,多菌株益生菌制剂比单菌株更有效,这可能增加益生菌黏附的机会。由于胃肠道的复杂性和变异性,益生菌的定殖可能有个体差异。迄今为止,尽管益生菌已经在运动领域显示出巨大的潜力,但大多数报道都是初步的前期研究,并且相关文献数量有限,菌株的剂量和确切的益处尚未完全优化,因此给运动员补充益生菌仍然是一个新兴领域。基于益生菌的基础作用,益生菌干预可能通过构建肠道微生态平衡,进而发挥促进运动员健康的作用,而目前其发挥保护作用的靶点、机制尚不明确。肠道菌群稳态、细菌产生的SCFA、肠黏膜屏障和免疫细胞的功能可能是未来的研究的方向。
[1]DREW M,VLAHOVICH N,HUGHES D,et al.Prevalence of illness,poor mental health and sleep quality and low energy availability prior to the 2016 Summer Olympic Games[J].British Journal of Sports Medicine,2018,52(1):47-53.
[2]ENGEBRETSEN L,SOLIGARD T,STEFFEN K,et al.Sports injuries and illnesses during the London Summer Olympic Games 2012[J].British Journal of Sports Medicine,2013,47(7):407-414.
[3]CASTELL L M,NIEMAN D C,BERMON S,et al.Exercise-induced illness and inflammation:Can immunonutrition and iron help?[J].International Journal of Sport Nutrition and Exercise Metabolism,2019,29(2):181-188.
[4]COSTA R,SNIPE R,KITIC C M,et al.Systematic review:Exercise-induced gastrointestinal syndrome-implications for health and intestinal disease[J].Alimentary Pharmacology&Therapeutics,2017,46(3):246-265.
[5]FASSARELLA M,BLAAK E E,PENDERS J,et al.Gut microbiome stability and resilience:Elucidating the response to perturbations in order to modulate gut health[J].Gut,2021,70(3):595-605.
[6]PARADA V D,De LA FUENTE M K,LANDSKRON G,et al.Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases [J].Frontiers in Immunology,2019,10:277.
[7]BARTON W,PENNEY N C,CRONIN O,et al.The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level[J].Gut,2018,67(4):625-633.
[8]KARL J P,MARGOLIS L M,MADSLIEN E H,et al.Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress[J].American Journal of Physiology.Gastrointestinal and Liver Physiology,2017,312(6):G559-G571.
[9]JAGER R,MOHR A E,PUGH J N.Recent advances in clinical probiotic research for sport [J].Current Opinion in Clinical Nutrition and Metabolic Care,2020,23(6):428-436.
[10]SIVAMARUTHI B S,KESIKA P,CHAIYASUT C.Effect of probiotics supplementations on health status of athletes[J].International Journal of Environmental Research and Public Health,2019,16(22):4469.
[11]LEE M C,HSU Y J,HO H H,et al.Lactobacillus salivarius Subspecies salicinius SA-03 is a new probiotic capable of enhancing exercise performance and decreasing fatigue[J].Microorganisms,2020,8(4):545.
[12]MOLLER G B,DA C G M,NICOLETTO B B,et al.Supplementation of probiotics and its effects on physically active individuals and athletes:Systematic review[J].International Journal of Sport Nutrition and Exercise Metabolism,2019,29(5):481-492.
[13]ESTRUEL-AMADES S,CAMPS-BOSSACOMA M,MASSOT-CLADERA M,et al.Alterations in the innate immune system due to exhausting exercise in intensively trained rats[J].Scientific Reports,2020,10(1):967-967.
[14]CLANCY R L,GLEESON M,COX A,et al.Reversal in fatigued athletes of a defect in interferon gamma secretion after administration of Lactobacillus acidophilus[J].British Journal of Sports Medicine,2006,40(4):351-354.
[15]LANCASTER G I,HALSON S L,KHAN Q,et al.Effects of acute exhaustive exercise and chronic exercise training on type 1 and type 2 T lymphocytes[J].Exercise Immunology Review,2004,10:91-106.
[16]WEST N P,PYNE D B,PEAKE J M,et al.Probiotics,immunity and exercise:A review[J].Exercise Immunology Review,2009,15:107-126.
[17]BATATINHA H,TAVARES-SILVA E,LEITE G,et al.Probiotic supplementation in marathonists and its impact on lymphocyte population and function after a marathon:A randomized placebo-controlled double-blind study[J].Scientific Reports,2020,10(1):18777.
[18]李鸿敏,周碎平,华岩.大强度运动对大鼠脾脏淋巴细胞、巨噬细胞、T 淋巴细胞亚群的影响[J].中国老年学杂志,2020,40(5):1051-1054.
LI H M,ZHOU S P,HUA Y.Effects of high intensity exercises on splenic lymphocyte,macrophage and T lymphocyte subsets in rats[J].Chinese Journal of Gerontology,2020,40(5):1051-1054.
[19]WALSH N P,GLEESON M,SHEPHARD R J,et al.Position statement.Part one:Immune function and exercise[J].Exercise Immunology Review,2011,17:6-63.
[20]HACKNEY A C,KOLTUN K J.The immune system and overtraining in athletes:Clinical implications[J].Acta Clinica Croatica,2012,51(4):633-641.
[21]赵振安,曹忠胜.microRNA 在调节变应性疾病Th1/Th2 平衡中作用的研究进展[J].中国免疫学杂志,2015,31(8):1151-1153.
ZHAO Z A,CAO Z S.Research progress on the role of microRNA in regulating Th1/Th2 balance in allergic diseases[J].Chinese Journal of Immunology,2015,31(8):1151-1153.
[22]MALDONADO-CONTRERAS A L,MCCORMICK B A.Intestinal epithelial cells and their role in innate mucosal immunity [J].Cell and Tissue Research,2011,343(1):5-12.
[23]FAHLMAN M M,ENGELS H J,HALL H.SIgA and upper respiratory syndrome during a college cross country season[J].Sports Medicine International Open,2017,1(6):E188-E194.
[24]NIEMAN D C,HENSON D A,DUMKE C L,et al.Relationship between salivary IgA secretion and upper respiratory tract infection following a 160-km race[J].The Journal of Sports Medicine and Physical Fitness,2006,46(1):158-162.
[25]PENAILILLO L,MAYA L,NINO G,et al.Salivary hormones and IgA in relation to physical performance in football[J].Journal of Sports Sciences,2015,33(20):2080-2087.
[26]NEVILLE V,GLEESON M,FOLLAND J P.Salivary IgA as a risk factor for upper respiratory infections in elite professional athletes[J].Medicine and Science in Sports and Exercise,2008,40 (7):1228-1236.
[27]VANCAMELBEKE M,VERMEIRE S.The intestinal barrier:A fundamental role in health and disease[J].Expert Review of Gastroenterology&Hepatology,2017,11(9):821-834.
[28]ODENWALD M A,TURNER J R.The intestinal epithelial barrier:A therapeutic target?[J].Nature Reviews.Gastroenterology&Hepatology,2017,14(1):9-21.
[29]PUGH J N,SPARKS A S,DORAN D A,et al.Four weeks of probiotic supplementation reduces GI symptoms during a marathon race[J].European Journal of Applied Physiology,2019,119(7):1491-1501.
[30]DE OLIVEIRA E P,BURINI R C,JEUKENDRUP A.Gastrointestinal complaints during exercise:Prevalence,etiology,and nutritional recommendations[J].Sports Medicine,2014,44(Suppl 1):S79-S85.
[31]GOMES J R,FREITAS J R,GRASSIOLLI S.Effects of physical exercise on the intestinal mucosa of rats submitted to a hypothalamic obesity condition[J].Anatomical Record,2016,299(10):1389-1396.
[32]HOLLAND A M,HYATT H W,SMUDER A J,et al.Influence of endurance exercise training on antioxidant enzymes,tight junction proteins,and inflammatory markers in the rat ileum[J].BMC Research Notes,2015,8:514.
[33]LARA-PADILLA E,GODINEZ-VICTORIA M,DRAGO-SERRANO M E,et al.Intermittent fasting modulates IgA levels in the small intestine under intense stress:A mouse model[J].Journal of Neuroimmunology,2015,285:22-30.
[34]SHING C M,PEAKE J M,LIM C L,et al.Effects of probiotics supplementation on gastrointestinal permeability,inflammation and exercise performance in the heat[J].European Journal of Applied Physiology,2014,114(1):93-103.
[35]ROBERTS J D,SUCKLING C A,PEEDLE G Y,et al.An exploratory investigation of endotoxin levels in novice long distance triathletes,and the effects of a multi-strain probiotic/prebiotic,antioxidant intervention[J].Nutrients,2016,8(11):733.
[36]COLBEY C,COX A J,PYNE D B,et al.Upper respiratory symptoms,gut health and mucosal immunity in athletes[J].Sports Medicine,2018,48(Suppl 1):65-77.
[37]COX A J,PYNE D B,SAUNDERS P U,et al.Oral administration of the probiotic Lactobacillus fermentum VRI-003 and mucosal immunity in endurance athletes[J].British Journal of Sports Medicine,2010,44(4):222-226.
[38]WEST N P,PYNE D B,CRIPPS A W,et al.Lactobacillus fermentum (PCC(R)) supplementation and gastrointestinal and respiratory-tract illness symptoms:A randomised control trial in athletes[J].Nutrition Journal,2011,10:30.
[39]GLEESON M,BISHOP N C,OLIVEIRA M,et al.Daily probiotic's (Lactobacillus casei Shirota) reduction of infection incidence in athletes[J].International Journal of Sport Nutrition and Exercise Metabolism,2011,21(1):55-64.
[40]MICHALICKOVA D,MINIC R,DIKIC N,et al.Lactobacillus helveticus Lafti L10 supplementation reduces respiratory infection duration in a cohort of elite athletes:A randomized,double-blind,placebo-controlled trial[J].Applied Physiology,Nutrition,and Metabolism,2016,41(7):782-789.
[41]STRASSER B,GEIGER D,SCHAUER M,et al.Probiotic supplements beneficially affect tryptophankynurenine metabolism and reduce the incidence of upper respiratory tract infections in trained athletes:A randomized,double-blinded,placebo-controlled trial[J].Nutrients,2016,8(11):752.
[42]HAYWOOD B A,BLACK K E,BAKER D,et al.Probiotic supplementation reduces the duration and incidence of infections but not severity in elite rugby union players[J].Journal of Science and Medicine in Sport,2014,17(4):356-360.
[43]KOMANO Y,SHIMADA K,NAITO H,et al.Efficacy of heat-killed Lactococcus lactis JCM 5805 on immunity and fatigue during consecutive high intensity exercise in male athletes:A randomized,placebo-controlled,double-blinded trial[J].Journal of the International Society of Sports Nutrition,2018,15(1):39.
[44]WEST N P,HORN P L,PYNE D B,et al.Probiotic supplementation for respiratory and gastrointestinal illness symptoms in healthy physically active individuals[J].Clinical Nutrition,2014,33(4):581-587.
[45]KEKKONEN R A,VASANKARI T J,VUORIMAA T,et al.The effect of probiotics on respiratory infections and gastrointestinal symptoms during training in marathon runners[J].International Journal of Sport Nutrition and Exercise Metabolism,2007,17(4):352-363.
[46]GLEESON M,BISHOP N C,STRUSZCZAK L.Effects of Lactobacillus casei Shirota ingestion on common cold infection and herpes virus antibodies in endurance athletes:A placebo-controlled,randomized trial[J].European Journal of Applied Physiology,2016,116(8):1555-1563.
[47]PYNE D B,WEST N P,COX A J,et al.Probiotics supplementation for athletes-clinical and physiological effects[J].European Journal of Sport Science,2015,15(1):63-72.
[48]LOLLO P C,CRUZ A G,MORATO P N,et al.Probiotic cheese attenuates exercise-induced immune suppression in Wistar rats[J].Journal of Dairy Science,2012,95(7):3549-3558.
[49]VAISBERG M,PAIXAO V,ALMEIDA E B,et al.Daily intake of fermented milk containing Lactobacillus casei Shirota (Lcs) modulates systemic and upper airways immune/inflammatory responses in marathon runners[J].Nutrients,2019,11(7):1678.
[50]PUMPA K L,MCKUNE A J,HARNETT J.A novel role of probiotics in improving host defence of elite rugby union athlete:A double blind randomised controlled trial[J].Journal of Science and Medicine in Sport,2019,22(8):876-881.
[51]THOMAS C M,VERSALOVIC J.Probiotics-host communication:Modulation of signaling pathways in the intestine[J].Gut Microbes,2010,1(3):148-163.
[52]KEMGANG T S,KAPILA S,SHANMUGAM V P,et al.Cross-talk between probiotic Lactobacilli and host immune system[J].Journal of Applied Microbiology,2014,117(2):303-319.
[53]GILL S K,TEIXEIRA A M,ROSADO F,et al.High-dose probiotic supplementation containing Lactobacillus casei for 7 days does not enhance salivary antimicrobial protein responses to exertional heat stress compared with placebo[J].International Journal of Sport Nutrition and Exercise Metabolism,2016,26(2):150-160.
[54]CARBUHN A F,REYNOLDS S M,CAMPBELL C W,et al.Effects of probiotic (Bifidobacterium longum 35624) supplementation on exercise performance,immune modulation,and cognitive outlook in division I female swimmers[J].Sports,2018,6(4):116.
[55]TOWNSEND J R,BENDER D,VANTREASE W C,et al.Effects of probiotic (Bacillus subtilis DE111) supplementation on immune function,hormonal status,and physical performance in division I baseball players[J].Sports,2018,6(3):70.
[56]MOREIRA A,KEKKONEN R,KORPELA R,et al.Allergy in marathon runners and effect of Lactobacillus GG supplementation on allergic inflammatory markers[J].Respiratory Medicine,2007,101 (6):1123-1131.
[57]MILES M P.Probiotics and gut health in athletes[J].Current Nutrition Reports,2020,9(3):129-136.
[58]LAMPRECHT M,BOGNER S,SCHIPPINGER G,et al.Probiotic supplementation affects markers of intestinal barrier,oxidation,and inflammation in trained men;a randomized,double-blinded,placebo-controlled trial[J].Journal of the International Society of Sports Nutrition,2012,9(1):45.
[59]GILL S K,ALLERTON D M,ANSLEY-ROBSON P,et al.Does short-term high dose probiotic supplementation containing Lactobacillus casei attenuate exertional-heat stress induced endotoxaemia and cytokinaemia?[J].International Journal of Sport Nutri tion and Exercise Metabolism,2016,26(3):268-275.
[60]MONDA V,VILLANO I,MESSINA A,et al.Exercise modifies the gut microbiota with positive health effects[J].Oxidative Medicine and Cellular Longevity,2017,2017:3831972.
[61]SCHMITZ L,FERRARI N,SCHWIERTZ A,et al.Impact of endurance exercise and probiotic supplementation on the intestinal microbiota:A cross-over pilot study[J].Pilot and Feasibility Studies,2019,5:76.
[62]AZAD M,SARKER M,LI T,et al.Probiotic species in the modulation of gut microbiota:An overview[J].BioMed Research International,2018,2018:9478630.
[63]CHAVES F M,BAPTISTA I L,SIMABUCO F M,et al.High-intensity-exercise-induced intestinal damage is protected by fermented milk supplemented with whey protein,probiotic and pomegranate(Punica granatum L.)[J].The British Journal of Nutrition,2018,119(8):896-909.
[64]AXELROD C L,BRENNAN C J,CRESCI G,et al.UCC118 supplementation reduces exercise-induced gastrointestinal permeability and remodels the gut microbiome in healthy humans[J].Physiological Reports,2019,7(22):e14276.
[65]DUNCAN S H,LOUIS P,FLINT H J.Lactate-utilizing bacteria,isolated from human feces,that produce butyrate as a major fermentation product[J].Applied and Environmental Microbiology,2004,70(10):5810-5817.
[66]OSMAN N,ADAWI D,AHRNE S,et al.Modulation of the effect of dextran sulfate sodium-induced acute colitis by the administration of different probiotic strains of Lactobacillus and Bifidobacterium[J].Digestive Diseases and Sciences,2004,49 (2):320-327.
[67]RODRIGUEZ-NOGALES A,ALGIERI F,GARRIDO-MESA J,et al.Differential intestinal anti-inflammatory effects of Lactobacillus fermentum and Lactobacillus salivarius in DSS mouse colitis:Impact on microRNAs expression and microbiota composition[J].Molecular Nutrition&Food Research,2017,61(11):1700144.
[68]UENO N,FUJIYA M,SEGAWA S,et al.Heatkilled body of Lactobacillus brevis SBC8803 ameliorates intestinal injury in a murine model of colitis by enhancing the intestinal barrier function[J].In flammatory Bowel Diseases,2011,17(11):2235-2250.
[69]MARTIN R,CHAMIGNON C,MHEDBI-HAJRI N,et al.The potential probiotic Lactobacillus rhamnosus CNCM I-3690 strain protects the intestinal barrier by stimulating both mucus production and cytoprotective response[J].Scientific Reports,2019,9(1):5398.
[70]WANG Y,GU Y,FANG K,et al.Lactobacillus acidophilus and Clostridium butyricum ameliorate colitis in murine by strengthening the gut barrier function and decreasing inflammatory factors[J].Beneficial Microbes,2018,9(5):775-787.
[71]GUO S,GILLINGHAM T,GUO Y,et al.Secretions of Bifidobacterium infantis and Lactobacillus acidophilus protect intestinal epithelial barrier function[J].Journal of Pediatric Gastroenterology and Nutrition,2017,64(3):404-412.
[72]KABEERDOSS J,DEVI R S,MARY R R,et al.Effect of yoghurt containing Bifidobacterium lactis Bb12 (R) on faecal excretion of secretory immunoglobulin A and human beta-defensin 2 in healthy adult volunteers[J].Nutrition Journal,2011,10:138.
[73]JAIN S,YADAV H,SINHA P R.Probiotic dahi containing Lactobacillus casei protects against Salmonella enteritidis infection and modulates immune response in mice [J].Journal of Medicinal Food,2009,12(3):576-583.
[74]RAWSON E S,MILES M P,LARSON-MEYER D E.Dietary supplements for health,adaptation,and recovery in athletes[J].International Journal of Sport Nutrition and Exercise Metabolism,2018,28(2):188-199.