1 姜黄素类化合物
姜黄素类化合物在桦木科、巴西豆科、马尾树科、蒟蒻薯科等多科植物中均有发现,主要从姜黄根茎(含量为2%~6%)中提取[1]。自古以来,中国和印度利用姜黄素进行着色以及香料制作,印度亦用其进行改善消化、消除蠕虫、缓解肠胃胀气、清洁肝脏[2]等。传统中药则将姜黄素用于行气破淤、活血止痛。在现代工业中,姜黄素被用于着色剂、酸碱指示剂、功能性食品以及膳食补充剂等领域。市场研究公司SPINS 数据显示[3],仅2019年,含姜黄提取物的冷热谷物产品分别增长928%和620%,市场销售总额近135 万美元。除谷物产品,在饮料产品、零食产品、乳制品中都可见姜黄提取物的身影。2020年美国植物委员会报告显示[4],姜黄提取物销售情况位列第4,产品主要解决免疫健康、关节健康、消化健康等方面存在的问题。姜黄素具有的健康效应引起人们的关注并带动姜黄产业的发展,解析其健康效应产生的物质基础和过程机制并最大化其健康效应,是姜黄素类化合物研究中非常重要的科学问题。
1.1 类别与结构
姜黄素类化合物具有一条庚烷长链和双苯环的基础结构,在此基础上,根据姜黄素类化合物庚烷母体长链上含氧取代基的种类和数目以及是否成环进一步分为单氧代型、双氧代型、三氧代型、吡喃取代型、呋喃取代型、大环醚型、二聚体型等7 个类型(图1)。一般而言,姜黄素类化合物是指姜黄素【1,7-双(4-羟基-3-甲氧基苯基)-1,6-庚二烯-3,5-二酮】、去甲氧基姜黄素【4-羟基肉桂酰基-(4-羟基-3-甲氧基肉桂酰基)甲烷】和双去甲氧基姜黄素【双-(4-羟基肉桂酰)甲烷】的混合物(图1)。它们具有相同的庚烷长链和双苯环结构,区别在于苯环上的甲氧基数目不同。其中,姜黄素含两个甲氧基,去甲氧基姜黄素含一个甲氧基,双去甲氧基姜黄素不含甲氧基,在它们的长链上存在着酮-烯醇式互变结构,以晶体形式和无定形分子形式存在时均为烯醇式结构,特殊情况下可向酮结构转变[5-6]。天然来源的典型姜黄素类化合物组成为52%~63%姜黄素、19%~27%去甲氧基姜黄素、18%~28%双去甲氧基姜黄素[7]。以姜黄素为例,分子结构以β-二酮结构中心对称,降低了分子极性,母体链中存在多个双键结构,这是使它成为一种高效抗氧化剂的前提,而两端的对位酚羟基也可增加其活性[8-10]。此外,姜黄素是一种天然的荧光标记物,在醇溶液和二甲基甲酰胺(DMF)作为潜在氢键供体时,荧光范围在536~560 nm 范围[11-12],而在极性溶液中其荧光光谱在408~540 nm 范围[13]。

图1 姜黄素类化合物分类及其3 种常见姜黄素类化合物的化学结构
Fig.1 Classification of curcuminoids and chemical structures of three kinds of major components of curcuminoids
1.2 姜黄素的稳定性及生物利用度
姜黄素难溶于水(小于50 μmol/L)[14],其水溶液在中性至碱性条件下不稳定,主要分解产物为阿魏酰甲烷(4-羟基-3-甲氧基肉桂酰甲烷)、阿魏酸(4-羟基-3-甲氧基肉桂酸)和香草醛(3-甲氧基-4-羟基苯甲醛)[15]。姜黄素是一种光敏性物质,在24 h 强光照射条件下姜黄素无水乙醇溶液被完全降解,其溶液由黄色转变为无色[16]。经口摄入后在胃液及小肠液溶解度低,其小肠透过性低[17],可被肠腔游离的还原酶、上皮细胞和肝脏代谢(Ⅰ相和Ⅱ相)并排除[18],最终导致其生物利用度极低。Perkins 等[19]进行小鼠体内实验,高效液相色谱法测定口服100 mg/kg 姜黄素后,小鼠血浆中仅有0.22 μg/mL 姜黄素;Yang 等[20]结合LC-MS/MS 方法测定口服500 mg/kg 姜黄素的大鼠血浆中姜黄素浓度,血药质量浓度仅达0.06 μg/mL。
针对上述姜黄素存在的稳定性差以及口服生物利用度低的问题,研究人员尝试了多种稳态递送体系对其进行包埋和递送[21-23]。前期研究[24]以高粱醇溶蛋白-羧甲基壳聚糖纳米颗粒对姜黄素进行包埋递送,改善了姜黄素在胃肠道的溶解度和细胞摄取效率。利用粉碎淀粉颗粒稳定的Pickering 乳液递送姜黄素[25],试验显示包封姜黄素的生物可及性比游离姜黄素高22.1%。Karade 等[26]制备抗坏血酸和姜黄素共递送的结肠定向微球,以抗坏血酸为pH 值调节剂可使姜黄素在结肠碱性环境中保持稳定,最终结肠释放的姜黄素生物利用度提高7 倍。
1.3 姜黄素的健康效应
姜黄素作为一种被广泛关注的活性物,临床耐受可达12 g/d 而无不良反应[27-28],是一种具备高效保健潜力的功能食品配料,常作为递送体系研究的模式物。姜黄素在体外和体内表现出多种生物学活性,如抗癌[29-31]、抗炎[29,32-33]、抗氧化[34-36]、抗HIV[37]、抗动脉粥样硬化[38-39]、抗糖尿病[40-41]、心血管疾病[42],降脂保肝[43-45],保护神经等。近年来,其对阿尔茨海默症等神经退行性疾病的预防作用受到人们的关注[46-48]。
首先,姜黄素显示出突出的体内抗氧化健康效应。活性氧(ROS)和其它自由基是机体正常代谢的副产物,可传导细胞信号和维持体内稳态。一旦体内ROS 水平急剧上升则可造成细胞损伤[49]。姜黄素可以在这种氧化应激过程中对机体起到保护作用,其健康效应归功于其抗氧化能力,包括清除自由基、过氧化氢和金属螯合等[50]。Hejazi 等[51]通过双盲试验评估姜黄素的抗氧化能力,患者以3 g/d 的剂量口服,试验结果显示超氧化物歧化酶(SOD)活性下降且血浆总抗氧化能力(TAC)增加。抗炎作用是姜黄素另一明确的、受到广泛研究的健康效应。研究表明,姜黄素能够抑制炎症相关的信号通路,包括核因子κB(NF-κB)、环氧合酶(COX)和脂氧合酶通路,从而降低促炎细胞因子水平和减少体内产生的前列腺素[52]。Desai 等[53]评估结肠靶向微丸递送姜黄素和环孢菌素对肠道炎症的干预效果,药效学研究结果表明,其在乙酸诱导的结肠炎症模型中有治疗效果且与环孢菌素显示出协同作用。错误折叠的淀粉样蛋白在体内累积是突触损伤和神经元沟通受损的主要原因之一,最终可发展成神经退行性疾病[54]。除上述抗炎机制,姜黄素可以透过血脑屏障(BBB)[55],抑制淀粉样蛋白的聚集和减轻其神经毒性作用[56]。此外,姜黄素可提高神经元生长因子(如NGF、BDNF、GDNF)的表达水平,这对减少突触损伤和神经元死亡具有重要作用[57]。Maiti 等[58]通过固体脂质纳米粒递送姜黄素发现其通过激活分子伴侣(如HSP70、HSP90、HSP60 和HSP40 等蛋白)起到神经保护作用。
姜黄素显著的、多样的健康效应被广泛报道,而这与其在物理稳定性差、上消化道生物利用度及药代动力学特征存在巨大反差,姜黄素健康效应的发挥机制是长期以来困扰科学家的重要科学问题。解析姜黄素的体内吸收代谢途径是解决上述科学问题的关键所在。
2 姜黄素的吸收代谢过程研究进展
2.1 姜黄素体内代谢转化过程
以回肠-盲肠为分界点,口腔、胃、十二指肠、空肠和回肠通常被统称为上消化道,盲肠、结肠、直肠被称为下消化道。姜黄素在体内的吸收代谢及分布情况一直以来被广泛研究,研究对象包括小鼠、大鼠以及人体(如表1所示)。从表1 结果可知,只有痕量姜黄素可以被上皮细胞吸收,并在肝脏和血浆中分布,而绝大多数无法吸收的姜黄素最终随着消化进程的推进进入结肠。研究表明,诸如儿茶素[59-61]、大豆异黄酮[62-65]、姜黄素等多种化合物由于体内代谢位点的不同,会发生不同的代谢反应并产生不同的代谢产物。姜黄素经口摄入后在上消化道被还原酶代谢发生还原反应,同时部分被吸收进入小肠上皮细胞,在上皮细胞作用下发生结合反应。虽然部分姜黄素可避免小肠首过效应进入肝脏,但仍会在肝脏处发生代谢生成极性更大的代谢产物并排出体外。由于姜黄素水溶性低和小肠透过性差,绝大部分姜黄素未经小肠消化直接进入结肠部位,经过结肠微生物及其相应酶系的羟基化、乙酰化、甲基化、还原、去甲氧基等作用,母体姜黄素被进一步代谢降解,其代谢产物可经过肠壁吸收进入体循环或随粪便排出体外(图2)。

图2 姜黄素体内吸收代谢过程示意图
Fig.2 Adsorption and metabolic pathways of curcumin in vivo
表1 姜黄素在体内的吸收分布研究(体内试验)
Table 1 Absorption and distribution of curcumin(derived from in vivo experiments)
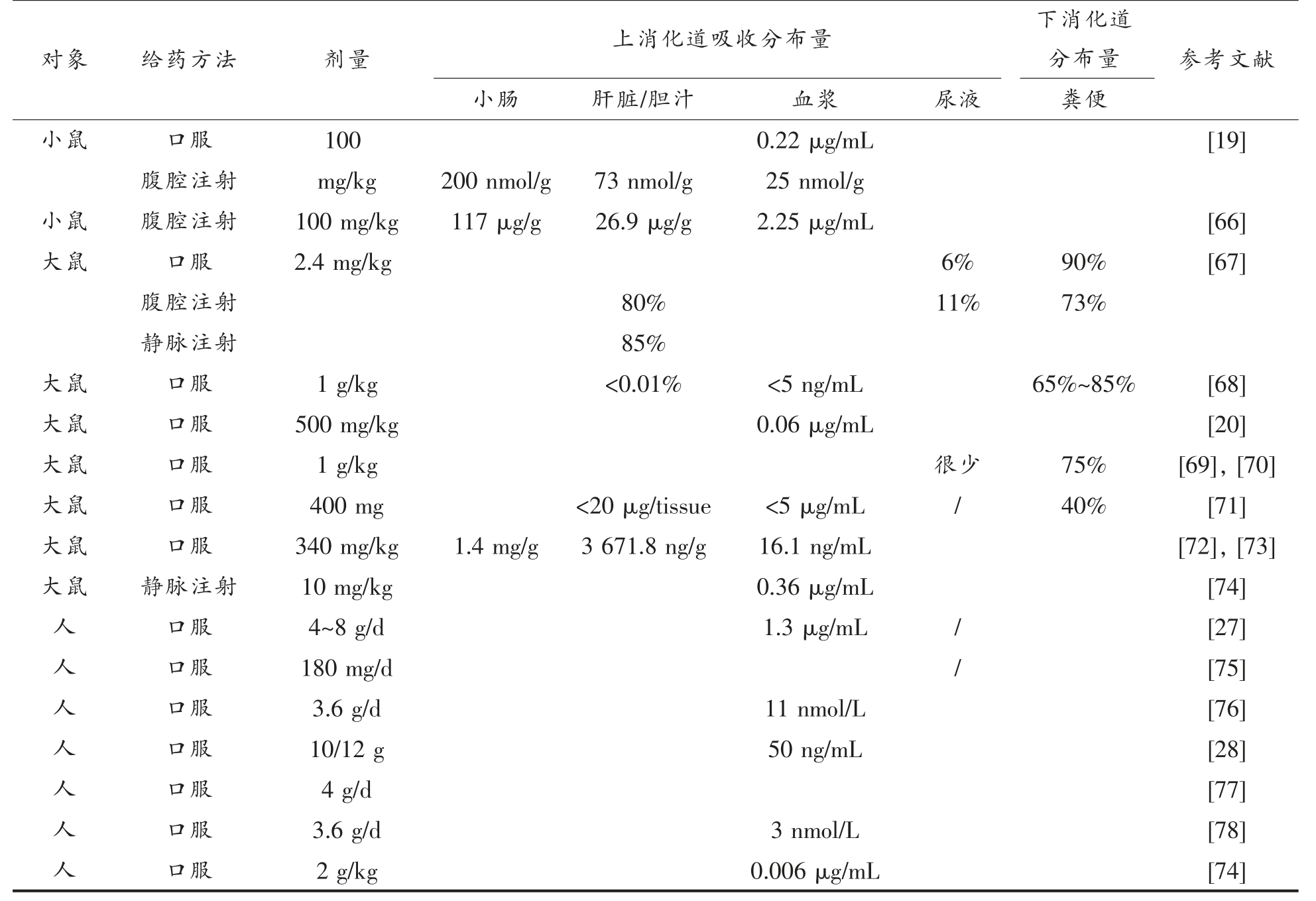
对象给药方口服法剂量上消化道吸收分布量下消化道分布量参考文献小肠肝脏/胆汁血浆尿液粪便小鼠1000.22 μg/mL[19]腹腔注射mg/kg200 nmol/g73 nmol/g25 nmol/g小鼠腹腔注射100 mg/kg117 μg/g26.9 μg/g2.25 μg/mL[66]大鼠口服2.4 mg/kg6%90%[67]腹腔注射80%11%73%静脉注射85%大鼠口服1 g/kg<0.01%<5 ng/mL65%~85%[68]大鼠口服500 mg/kg0.06 μg/mL[20]大鼠口服1 g/kg很少75%[69],[70]大鼠口服400 mg<20 μg/tissue<5 μg/mL/40%[71]大鼠口服340 mg/kg1.4 mg/g3 671.8 ng/g16.1 ng/mL[72],[73]大鼠静脉注射10 mg/kg0.36 μg/mL[74]人口服4~8 g/d1.3 μg/mL/[27]人口服180 mg/d/[75]人口服3.6 g/d11 nmol/L[76]人口服10/12 g50 ng/mL[28]人口服4 g/d[77]人口服3.6 g/d3 nmol/L[78]人口服2 g/kg0.006 μg/mL[74]
2.2 姜黄素在上消化道的代谢转化
姜黄素在上消化道主要发生氧化反应,还原反应和结合反应(图3)。氧化反应生成双环戊二酮衍生物,其有两种产生途径:一种是姜黄素发生自氧化,另一种是通过脂肪氧化酶(LOX)和COX的催化作用产生,母链上羟基的氢脱去并发生环化和氧合,从而产生醌甲基和庚二酮链中的离域自由基,随后醌甲基继续发生水合和重排反应,最终姜黄素转化成双环戊二酮衍生物。虽然在体内有两种途径可以产生双环戊二酮衍生物,但是酶催化氧化产生的速率更快,在LOX 和COX 含量高的细胞里,双环戊二酮衍生物的含量通常高于其它代谢产物[10,79]。

图3 姜黄素在上消化道的代谢转化途径
Fig.3 Metabolic transformation pathways of curcumin in the upper gastrointestinal tract
还原反应是姜黄素在上消化道中发生的更为普遍的反应,还原酶存在时姜黄素即可能被还原。由于存在多个双键,姜黄素能根据发生氧化双键的个数分别被氧化为二氢、四氢、六氢和八氢代谢产物,以姜黄素为例,四氢姜黄素和六氢姜黄素的代谢水平较高,血浆中以四氢姜黄素的形式存在为主[80]。值得注意的是,肠道内姜黄素及其还原产物在进入肠道上皮细胞和到达肝脏之后很容易发生结合反应,主要分为葡萄糖醛酸化和硫酸化两种,可分别发生或者共同发生。以葡萄糖醛酸化为例,在β-葡萄糖醛酸苷酶的作用下姜黄素主链羟基上或是苯环上的氢被葡萄糖醛酸取代,增加化合物的极性便于溶解和排出体外。多项研究显示[81-83],六氢姜黄素-葡萄糖醛酸结合物是姜黄素在体液、器官和细胞中的主要代谢物。
3 姜黄素与肠道菌群的互作过程
长期以来研究人员普遍认为上消化道是消化代谢的主要场所,下消化道主要负责水分、电解质的重吸收和排泄,然而针对提高姜黄素上消化道吸收所作出的尝试并未取得重大成效。近年来,学界开始意识到姜黄素极有可能在分布水平较高的下消化道发挥作用[66],包括代谢成具有更高活性的代谢物[84]和调节肠道菌群区系,从而直接或间接影响其在体内表现出的健康效应。
3.1 姜黄素在下消化道的代谢转化
相较于上消化道代谢产物的种类,姜黄素经下消化道肠道菌群代谢所产生的代谢产物更为丰富。其主要反应包括甲基化、去甲基化、羟基化、去羟基化、还原和去甲氧基化等反应途径,基本代谢产物如图4所示。值得注意的是,不同反应之间可以相互叠加,从而产生一些含量水平较低、结构更为复杂的次级代谢产物。例如,去甲基姜黄素和双去甲氧基姜黄素可以在人类结肠代谢产物中发现[85],它们可提高β-淀粉样蛋白降解酶的活性,减少β-淀粉样蛋白在海马体和大脑皮层中蓄积,而母体姜黄素则不能。姜黄素的两个苯环上各有一个甲氧基,当-OCH3 被替代成-H 属于去甲氧基化,当甲氧基上的-CH3 被肠道菌群代谢转换为-H,则为去甲基化过程;与去甲基化不同的是,有研究显示[86-87]甲基化反应可以发生在姜黄素母链的醇羟基上,也可以发生在苯环上的酚羟基上,存在较多可能。羟基化是发生在姜黄素母链上的代谢途径,肠道菌群在母链的碳原子上引入一个氧原子,从而形成一个羟基或者进一步生成羰基,而去羟基化则是更倾向于脱去母链上的羟基。因为还原酶在体内大量存在,结肠也不例外,所以姜黄素及其代谢产物在进入结肠后也可以继续发生还原反应。

图4 姜黄素在下消化道的代谢途径
Fig.4 Metabolic transformation pathways of curcumin in the lower gastrointestinal tract
肠道菌群对姜黄素的生物转化研究主要应用人类粪便浆液以及单一细菌菌株两类试验模型。虽然两种模型的分析方法相近,但通过人类粪便浆液模型所产生的代谢产物种类更为丰富,足见菌群多样性对代谢产物种类和水平的影响。姜黄素的肠道菌群代谢产物种类繁多(表2),以阿魏酸、四氢姜黄素、八氢姜黄素、去甲氧基姜黄素、双去甲氧基姜黄素等为主。已有研究证明这些代谢产物具有比姜黄素更高的生物活性,并可以通过不同途径影响姜黄素的炎症治疗和保护神经效果[88-91]。
表2 肠道菌群对姜黄素类化合物的代谢研究
Table 2 Studies on the metabolism of curcumins by gut microbiota

模型原型物菌群代谢产物分析方法人类粪便浆液姜黄素、去甲氧基姜黄素、双去甲氧基姜黄素四氢姜黄素、二氢阿魏酸、1-(4-羟基-3-甲氧基苯基)-2-丙醇超高压液相色谱(UHPLC)线性离子阱质谱姜黄素23 种去甲基化、还原、羟基化和乙酰化、或这些的组合的产物超高效液相色谱结合四极杆飞行时间质谱(UPLC-Q-TOF MS)细菌菌株布劳特氏菌属MRGPMF1去甲基姜黄素、双去甲基姜黄素费格森埃希菌和大肠杆菌姜黄素、去甲氧基姜黄素、双去甲氧基姜黄素二氢姜黄素、四氢姜黄素、阿魏酸、姜黄素-L-半胱氨酸巨 大 芽 孢 杆 菌DCMB-002姜黄素、去甲氧基姜黄素、双去甲氧基姜黄素姜黄素7 种去甲基化、还原、羟基化和去甲氧基化代谢产物高效液相色谱-四极杆飞行时间串联质谱
3.2 姜黄素对肠道菌群的调节作用
姜黄素进入结肠后不仅会受到肠道菌群影响,同时也可以影响肠道菌群结构,这种结构上的调整可间接影响姜黄素健康效应的发挥[92]。肠道菌群结构表现为菌群的丰富度、多样性及表达水平。保持肠道有益菌群水平处于优势有助于人体健康,而致病菌一旦过度繁殖会导致机体产生系统性疾病,尽管生物失调和疾病之间的直接因果关系尚不明确,但它们之间相互影响的关系越来越被学界认可[93-94]。研究表明,姜黄素的摄入往往会调节结肠内肠道菌群的丰富度和多样性[92,95]。Shen 等[92]探究了口服姜黄素对C57BL/6 小鼠肠道微生物区系的调节作用,普雷沃氏菌科、理研菌科和类杆菌科等代表性菌属的表达水平显著下降。Sun 等[96]研究了姜黄素与APP/PS1 双转基因小鼠肠道微生物区系的相互作用,与对照组相比,姜黄素处理组类杆菌科、普雷沃氏菌科和乳杆菌科的丰度显著下降,而理研菌的丰度显著提升。Sreng等[97]发现姜黄素处理影响到不同的菌属,显著增加类杆菌科而减少了理研菌科的丰度。尽管不同试验对肠道菌群相同菌属的影响可能不同,但姜黄素能显著影响的菌群种类已经逐渐清晰,这种影响与姜黄素健康效应之间的关系有望被理清。其次,口服姜黄素可提高产短链脂肪酸有益菌群水平,而短链脂肪酸对心血管疾病、代谢疾病、神经退行性疾病和免疫疾病[98-99]等领域都具有重要贡献。Li 等[100]对姜黄素给药后结肠内的乙酸、丙酸、丁酸、异丁酸、戊酸、异戊酸和己酸进行定量分析,除丁酸外基本回到正常表达水平,丙酸表达水平甚至超过对照组。
姜黄素在下消化道所引起的变化让研究人员不得不重新审视姜黄素发挥健康效应的作用途径。低生物利用度和广泛的健康效应之间的矛盾可能需要通过姜黄素与肠道菌群区系之间的相互作用进行阐释,高活性的姜黄素代谢物直接引起机体的健康效应,而肠道菌群结构的调节影响有益、有害代谢产物和疾病相关因子的产生,从而间接促进健康效应的发挥。
4 结论
姜黄素的诸多健康效应如抗炎、抗氧化、抗神经退行性疾病等都已经被证实。令人不解的是,姜黄素在体内由于溶解性差和透过性差导致有效浓度低下,这与其广泛的健康效应相违背,以至于学界对其发挥功效的物质基础和作用途径存在较大争议。一直以来对姜黄素的研究都集中于上消化道,一定程度上是由于人们过度区分上下消化道的功能作用,从而忽略了下消化道可能存在的代谢和吸收。随着肠道菌群被视为人体的“第二大脑”,包括姜黄素在内的植物化学活性物质是否可通过结肠发挥健康效应逐渐得到重视。目前姜黄素的口服递送增效体系的设计目标旨在增强上消化道的吸收从而提高生物利用度,然而这对姜黄素的功效发挥不一定是有利的。在下消化道与肠道菌群相互作用更可能是其发挥健康效应的基础,通过结肠靶向递送体系规避上消化道吸收的同时提高下消化道的释放,是探究姜黄素健康效应作用机制的有效手段。
[1]NIRMAL B K,MINOO D,GEETHA S P,et al.Biotechnology of turmeric and related species turmeric:The genus curcuma[M].Boca Raton:CRC Press,2007:107-127.
[2]BHOWMIK D,CHIRANJIB,KUMAR K P S,et al.Turmeric:A herbal and traditional medicine[J].Archives of Applied Science Research,2009,1(2):86-108.
[3]SMITH T,GILLESPIE M,ECKL V,et al.Herbal supplement sales in US increase by 9.4% in 2018[EB/OL].The Journal of American Botanical Council,(2019-08-12)[2021-09-02].http://cms.herbalgram.org/herbalgram/issue123/files/HG123-HMR.pdf.
[4]SMITH T,MAY G,ECKL V,et al.US Sales of Herbal Supplements Increase by 8.6% in 2019[EB/OL].The Journal of American Botanical Council,(2020-07-24)[2021-09-02].http://herbalgram.org/re sources/herbalgram/issues/127/table -of -contents/herbalgram-127-herb-market-report-american-botanical-council/.
[5]FANG J G,LU J,ARNE H.Thioredoxin reductase is irreversibly modified by curcumin:A novel molecular mechanism for its anticancer activity[J].Journal of Biological Chemistry,2005,280(26):25284-25290.
[6]MARCU M G,YUN JIN J,SUNMIN L,et al.Curcumin is an inhibitor of p300 histone acetylatransferase[J].Medicinal Chemistry,2006,2(2):169-174.
[7]MADSEN B,HIDALGO GARCIA V,HERNANDEZ VERA L.Purification process for improving total yield of curcuminoid colouring agent[J].Bioorg Med Chem Lett,2015,25(22):5067-5071.
[8]狄建彬,顾振纶,赵笑东,等.姜黄素的抗氧化和抗炎作用研究进展[J].中草药,2010,41(5):854-857.DI J B,GU Z L,ZHAO X D,et al.Advances in studies on antioxidant and anti-inflammation of curcumin[J].Chinese Traditional and Herbal Drugs,2010,41(5):854-857.
[9]吴宏伟.基于代谢组学的姜黄、郁金寒热药性差异研究[D].北京:中国中医科学院,2011.WU H W.Studies on warm and cold nature of JiangHuang and YuJin based on metabonomics[D].Beijing:China Academy of Chinese Medical Sciences,2011.
[10]MANFRED M,ERIKA P,SCHULZ S I,et al.Curcumin uptake and metabolism [J].Biofactors,2013,39(1):14-20.
[11]LUCA N,ROBERTA P,ALESSANDRA A,et al.Role of H-bond formation in the photoreactivity of curcumin[J].Spectroscopy An International Journal,2008,22(2/3):187-198.
[12]LEE W H,LOO C Y,BEBAWY M,et al.Curcumin and its derivatives:Their application in neuropharmacology and neuroscience in the 21st century[J].Curr Neuropharmacol,2013,11(4):338-378.
[13]PRIYADARSINI K I.The chemistry of curcumin:From extraction to therapeutic agent[J].Molecules,2014,19(12):20091-20112.
[14]MOHANTY C,DAS M,SAHOO S K.Emerging role of nanocarriers to increase the solubility and bioavailability of curcumin [J].Expert Opinion on Drug Delivery,2012,9(11):1347-1364.
[15]WANG Y J,PAN M H,CHENG A L,et al.Stability of curcumin in buffer solutions and characterization of its degradation products[J].J Pharm Biomed Anal,1997,15(12):1867-1876.
[16]陶慧,余楚钦,黄劲恒,等.姜黄素的增溶及稳定性研究[J].食品与发酵工业,2016,42(8):160-165.TAO H,YU C Q,HUANG J H,et al.The solubility and stability of curcumin[J].Food and Fermentation Industries,2016,42(8):160-165.
[17]WAHLANG B,PAWAR Y B,BANSAL A K.Identification of permeability-related hurdles in oral delivery of curcumin using the Caco-2 cell model[J].European Journal of Pharmaceutics And Biopharmaceutics,2011,77(2):275-282.
[18]MICHELE DEI C,RICCARDO G.Dietary curcumin:Correlation between bioavailability and health potential[J].Nutrients,2019,11(9):2147.
[19]PERKINS S,VERSCHOYLE R D,HILL K,et al.Chemopreventive efficacy and pharmacokinetics of curcumin in the min/+ mouse,a model of familial adenomatous polyposis[J].Cancer Epidemiol Biomarkers Prev,2002,11(6):535-540.
[20]YANG K Y,LIN L C,TSENG T Y,et al.Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC-MS/MS[J].J Chromatogr B Analyt Technol Biomed Life Sci,2007,853(1/2):183-189.
[21]KHARAT M,MCCLEMENTS D J.Recent advances in colloidal delivery systems for nutraceuticals:A case study - delivery by design of curcumin[J].J Colloid Interface Sci,2019,557(1):506-518.
[22]SAHEB M,FEREYDOUNI N,NEMATI S,et al.Chitosan-based delivery systems for curcumin:A review of pharmacodynamic and pharmacokinetic aspects[J].J Cell Physiol,2019,234(8):12325-12340.
[23]IPAR V S,DSOUZA A,DEVARAJAN P V.Enhancing curcumin oral bioavailability through nanoformulations [J].Eur J Drug Metab Pharmacokinet,2019,44(4):459-480.
[24]XIAO J,NIAN S,HUANG Q R.Assembly of kafirin/carboxymethyl chitosan nanoparticles to enhance the cellular uptake of curcumin[J].Food Hydrocolloids,2015,51(1):166-175.
[25]LU X X,ZHU J Y,PAN Y J,et al.Assessment of dynamic bioaccessibility of curcumin encapsulated in milled starch particle stabilized Pickering emulsions using TNO's gastrointestinal model [J].Food Funct,2019,10(5):2583-2594.
[26]KARADE P G,JADHAV N R.Colon targeted curcumin microspheres laden with ascorbic acid for bioavailability enhancement[J].Journal of Microencapsulation,2018,35(4):372-380.
[27]CHENG A,HSU C,LIN J,et al.Phase I clinical trial of curcumin,a chemopreventive agent,in patients with high-risk or pre-malignant lesions[J].Anticancer Research,2001,21(4B):2895-2900.
[28]LAO C D,IV M T R,NORMOLLE D,et al.Dose escalation of a curcuminoid formulation [J].Bmc Complementary & Alternative Medicine,2006,6(1):1-4.
[29]FADUS M C,LAU C,BIKHCHANDANI J,et al.Curcumin:An age-old anti-inflammatory and antineoplastic agent[J].Journal of Traditional & Complementary Medicine,2017,7(3):339-346.
[30]KUNNUMAKKARA A B,PREETHA A,AGGARWAL B B.Curcumin inhibits proliferation,invasion,angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins[J].Cancer Letters,2008,269(2):199-225.
[31]LEU T H,MAA M C.The molecular mechanisms for the antitumorigenic effect of curcumin[J].Curr Med Chem Anticancer Agents,2002,2(3):357-370.
[32]AGGARWAL B B,HARIKUMAR K B.Potential therapeutic effects of curcumin,the anti-inflammatory agent,against neurodegenerative,cardiovascular,pulmonary,metabolic,autoimmune and neoplastic diseases[J].International Journal of Biochemistry &Cell Biology,2009,41(1):40-59.
[33]ZHANG X X,WU J,YE B,et al.Protective effect of curcumin on TNBS-induced intestinal inflammation is mediated through the JAK/STAT pathway[J].Bmc Complementary & Alternative Medicine,2016,16(1):299.
[34]MASUDA T,MAEKAWA T,HIDAKA K,et al.Chemical studies on antioxidant mechanism of curcumin:Analysis of oxidative coupling products from curcumin and linoleate [J].J Agric Food Chem,2001,49(5):2539-2547.
[35]MIAO M,GUO L,TIAN S,et al.Effects of curcumin on antioxidation in diabetic rats[J].Pakistan Journal of Pharmaceutical Sciences,2015,28(1 Suppl):371-373.
[36]WEI Q Y,CHEN W F,ZHOU B,et al.Inhibition of lipid peroxidation and protein oxidation in rat liver mitochondria by curcumin and its analogues[J].Biochimica Et Biophysica Acta General Subjects,2006,1760(1):70-77.
[37]JORDAN W C,DREW C R.Curcumin - A natural herb with anti-HIV activity[J].Journal of the National Medical Association,1996,88(6):333.
[38]OLSZANECKI R,JAWIE N J,GAJDA M,et al.Effect of curcumin on atherosclerosis in apoE/LDLRdouble knockout mice[J].Journal of Physiology &Pharmacology,2005,56(4):627-635.
[39]SHIN S K,HA T Y,MCGREGOR R A,et al.Long-term curcumin administration protects against atherosclerosis via hepatic regulation of lipoprotein cholesterol metabolism[J].Molecular Nutrition & Food Research,2011,55(12):1829-1840.
[40]SOMLAK C,SUTHEE R,RATAYA L,et al.Curcumin extract for prevention of type 2 diabetes[J].Diabetes Care,2012,35(11):2121-2127.
[41]WEISBERG S P,RUDOLPH L,TORTORIELLO D V.Dietary curcumin significantly improves obesityassociated inflammation and diabetes in mouse models of diabesity[J].Endocrinology,2008,149(7):3549-3558.
[42]QING H Y,DONG Q W,CHANG C C,et al.Curcumin ameliorates left ventricular function in rabbits with pressure overload:Inhibition of the remodeling of the left ventricular collagen network associated with suppression of myocardial tumor necrosis factor-alpha and matrix metalloproteinase-2 exp[J].Biological & Pharmaceutical Bulletin,2004,27(2):198-202.
[43]PARK E J,JEON C H,KO G,et al.Protective effect of curcumin in rat liver injury induced by carbon tetrachloride[J].Journal of Pharmacy & Pharmacology,2000,52(4):437-440.
[44]SALAHSHOOR M,MOHAMADIAN S,KAKABARAEI S,et al.Curcumin improves liver damage in male mice exposed to nicotine[J].Journal of Traditional & Complementary Medicine,2016,6(2):176-183.
[45]ZHAO Y L,MA X,WANG J B,et al.Curcumin protects against CCl4-induced liver fibrosis in rats by inhibiting HIF-1α through an ERK-dependent pathway[J].Molecules,2014,19(11):18767-18780.
[46]CHEN M,DU Z Y,ZHENG X,et al.Use of curcumin in diagnosis,prevention,and treatment of Alzheimer's disease [J].Neural Regeneration Research,2018,13(4):742-752.
[47]BAUM L,NG A.Curcumin interaction with copper and iron suggests one possible mechanism of action in Alzheimer's disease animal models[J].Journal of Alzheimers Disease,2004,6(4):367-377.
[48]RINGMAN J M,FRAUTSCHY S A,COLE G M,et al.A potential role of the curry spice curcumin in Alzheimer's disease[J].Current Alzheimer Research,2005,2(2):131-136.
[49]BRIEGER K,SCHIAVONE S,MILLER F J,et al.Reactive oxygen species:From health to disease[J].Swiss Med Wkly,2012,142(3334):w13659.
[50]AK T,GÜLÇIN I.Antioxidant and radical scavenging properties of curcumin[J].Chem Biol Interact,2008,174(1):27-37.
[51]HEJAZI J,RASTMANESH R,TALEBAN F A,et al.Effect of curcumin supplementation during radiotherapy on oxidative status of patients with prostate cancer:A double blinded,randomized,placebocontrolled study[J].Nutr Cancer,2016,68(1):77-85.
[52]BURGE K,GUNASEKARAN A,ECKERT J,et al.Curcumin and intestinal inflammatory diseases:Molecular mechanisms of protection[J].Int J Mol Sci,2019,20(8):1912.
[53]DESAI N,MOMIN M.Colon targeted bioadhesive pellets of curcumin and cyclosporine for improved management of inflammatory bowel disease[J].Drug Deliv Transl Res,2020,10(5):1288-1301.
[54]DUGGER B N,DICKSON D W.Pathology of neurodegenerative diseases[J].Cold Spring Harb Perspect Biol,2017,9(7):a028035.
[55]LICZBINSKI P,MICHAŁOWICZ J,BUKOWSKA B.Molecular mechanism of curcumin action in signaling pathways:Review of the latest research[J].Phytother Res,2020,34(8):1992-2005.
[56]MAITI P,DUNBAR G L.Use of curcumin,a natural polyphenol for targeting molecular pathways in treating age - Related neurodegenerative diseases[J].Int J Mol Sci,2018,19(6):1637.
[57]GUPTA S C,PRASAD S,KIM J H,et al.Multitargeting by curcumin as revealed by molecular interaction studies[J].Nat Prod Rep,2011,28(12):1937-1955.
[58]MAITI P,DUNBAR G L.Comparative neuroprotective effects of dietary curcumin and solid lipid curcumin particles in cultured mouse neuroblastoma cells after exposure to Aβ42[J].Int J Alzheimers Dis,2017,2017(1):4164872.
[59]ACTIS-GORETTA L,LEVEQUES A,REIN M,et al.Intestinal absorption,metabolism,and excretion of(-)-epicatechin in healthy humans assessed by using an intestinal perfusion technique[J].American Journal of Clinical Nutrition,2013,98(4):924-933.
[60]ULLMANN U,HALLER J,DECOURT J P,et al.A single ascending dose study of epigallocatechin gallate in healthy volunteers[J].Journal of International Medical Research,2003,31(2):88-101.
[61]ZHOU Y,ZHANG N,ARIKAWA A Y,et al.Inhibitory effects of green tea polyphenols on microbial metabolism of aromatic amino acids in humans revealed by metabolomic analysis [J].Metabolites,2019,9(5):96.
[62]HEINONEN S M,HOIKKALA A,WÄHÄLÄ K,et al.Metabolism of the soy isoflavones daidzein,genistein and glycitein in human subjects.:Identification of new metabolites having an intact isoflavonoid skeleton[J].Journal of Steroid Biochemistry& Molecular Biology,2003,87(4/5):285-299.
[63]LEE D H,KIM M J,AHN J,et al.Nutrikinetics of isoflavone metabolites after fermented soybean product(Cheonggukjang)ingestion in ovariectomized mice [J].Molecular Nutrition & Food Research,2017,61(12):1700322.
[64]NAOKO S,JUN K,HIROSHI K.Dietary and microbial metabolites in the regulation of host immunity[J].Frontiers in Microbiology,2017,8(1):2171.
[65]XU X F,LI X H,LIANG X R.Application of ultra-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry in identification of three isoflavone glycosides and their corresponding metabolites[J].Rapid Communications in Mass Spectrometry,2018,32(3):262-268.
[66]PAN M H,HUANG T M,LIN J K.Biotransformation of curcumin through reduction and glucuronidation in mice[J].Drug Metabolism & Disposition,1999,27(4):486-494.
[67]HOLDER G M,PLUMMER J L,RYAN A J.The metabolism and excretion of curcumin(1,7-bis-(4-hydroxy -3 -methoxyphenyl)-1,6 -heptadiene -3,5 -dione)in the rat[J].Xenobiotica,1978,8(12):761-768.
[68]WAHLSTRÖM B,BLENNOW G.A study on the fate of curcumin in the rat[J].Acta Pharmacol Toxicol(Copenh),1978,43(2):86-92.
[69]AGGARWAL B B,SUNDARAM C,MOSLEY C A,et al.The molecular targets and therapeutic uses of curcumin in health and disease[M].Berlin:Springer Science & Business Media,2007:453-470.
[70]SHARMA R A,STEWARD W P,GESCHER A J.Pharmacokinetics and pharmacodynamics of curcumin[J].Advances in Experimental Medicine & Biology,2007,595(6):453.
[71]RAVINDRANATH V,CHANDRASEKHARA N.Absorption and tissue distribution of curcumin in rats[J].Toxicology,1980,16(3):259-265.
[72]MARCZYLO T H,VERSCHOYLE R D,COOKE D N,et al.Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine[J].Cancer Chemotherapy & Pharmacology,2007,60(2):171-177.
[73]MARCZYLO T H,STEWARD W P,GESCHER A J.Rapid analysis of curcumin and curcumin metabolites in rat biomatrices using a novel ultraperformance liquid chromatography(UPLC)method[J].J Agric Food Chem,2009,57(3):797-803.
[74]SHOBA G,JOY D,JOSEPH T,et al.Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers [J].Planta Medica,1998,64(4):353-356.
[75]SHARMA R A,MCLELLAND H R,HILL K A,et al.Pharmacodynamic and pharmacokinetic study of oral curcuma extract in patients with colorectal can cer[J].Clinical Cancer Research,2001,7(7):1894-1900.
[76]SHARMA R A,EUDEN S A,PLATTON S L,et al.Phase I clinical trial of oral curcumin:Biomarkers of systemic activity and compliance[J].Clinical Cancer Research,2004,10(20):6847.
[77]CARROLL R E,BENYA R V,TURGEON D K,et al.Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia[J].Cancer Prevention Research,2011,4(3):354-364.
[78]GARCEA G,JONES D J L,SINGH R,et al.Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration[J].Br J Cancer,2004,90(5):1011-1015.
[79]GRIESSER M,PISTIS V,SUZUKI T,et al.Autoxidative and cyclooxygenase-2 catalyzed transformation of the dietary chemopreventive agent curcumin[J].Journal of Biological Chemistry,2011,286(2):1114-1124.
[80]PETERSON C T,VAUGHN A R,SHARMA V,et al.Effects of turmeric and curcumin dietary supplementation on human gut microbiota:A double -blind,randomized,placebo-controlled pilot study[J].Journal of Evidence -Based Integrative Medicine,2018,23(1):1-8.
[81]HOEHLE S I,PFEIFFER E,SOLYOM A M,et al.Metabolism of curcuminoids in tissue slices and subcellular fractions from rat liver[J].Journal of Agricultural and Food Chemistry,2006,54(3):756-764.
[82]IRESON C,ORR S,JONES D J L,et al.Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo,and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production[J].Cancer Research,2001,61(3):1058-1064.
[83]IRESON C R,JONES D J L,ORR S,et al.Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine[J].Cancer Epidemiology Biomarkers & Prevention,2002,11(1):105-111.
[84]DI MEO F,MARGARUCCI S,GALDERISI U,et al.Curcumin,gut microbiota,and neuroprotection[J].Nutrients,2019,11(10):2426.
[85]BURAPAN S,KIM M,HAN J.Curcuminoid demethylation as an alternative metabolism by human intestinal microbiota[J].J Agric Food Chem,2017,65(16):3305-3310.
[86]冯生光.姜黄素的稳定性、降解产物及大鼠粪便中代谢产物研究[D].沈阳:沈阳药科大学,2008.FENG S G.Study on stability,degradation products and metabolites in rat feces of curcumin [D].Shenyang:Shenyang Pharmaceutical University,2008.
[87]LOU Y,ZHENG J,HU H,et al.Application of ultra -performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry to identify curcumin metabolites produced by human intestinal bacteria[J].Journal of Chromatography B Analytical Technologies in the Biomedical & Life Sciences,2015,985:38-47.
[88]SINGH C,BORTOLATO M,BALI N,et al.Cognitive abnormalities and hippocampal alterations in monoamine oxidase A and B knockout mice[J].Proc Natl Acad Sci U S A,2013,110(31):12816-12821.
[89]ZENG Y C,QIU F,LIU Y,et al.Isolation and identification of phase 1 metabolites of demethoxycurcumin in rats[J].Drug Metab Dispos,2007,35(9):1564-1573.
[90]LUO D D,CHEN J F,LIU J J,et al.Tetrahydrocurcumin and octahydrocurcumin,the primary and final hydrogenated metabolites of curcumin,possess superior hepatic-protective effect against acetaminophen-induced liver injury:Role of CYP2E1 and Keap1-Nrf2 pathway[J].Food Chem Toxicol,2019,123(1):349-362.
[91]VELDMAN E R,JIA Z,HALLDIN C,et al.Amyloid binding properties of curcumin analogues in Alzheimer's disease postmortem brain tissue[J].Neurosci Lett,2016,630(1):183-188.
[92]SHEN L,LIU L,JI H F.Regulative effects of curcumin spice administration on gut microbiota and its pharmacological implications[J].Food Nutr Res,2017,61(1):1361780.
[93]NI J,WU G D,ALBENBERG L,et al.Gut microbiota and IBD:Causation or correlation?[J].Nat Rev Gastroenterol Hepatol,2017,14(10):573-584.
[94]QUIGLEY E M M.Microbiota-brain-gut axis and neurodegenerative diseases[J].Curr Neurol Neurosci Rep,2017,17(12):94.
[95]FENG W H,WANG H D,ZHANG P Z,et al.Modulation of gut microbiota contributes to curcumin-mediated attenuation of hepatic steatosis in rats[J].Biochim Biophys Acta Gen Subj,2017,1861(7):1801-1812.
[96]SUN Z Z,LI X Y,WANG S,et al.Bidirectional interactions between curcumin and gut microbiota in transgenic mice with Alzheimer's disease[J].Appl Microbiol Biotechnol,2020,104(8):3507-3515.
[97]SRENG N,CHAMPION S,MARTIN J C,et al.Resveratrol-mediated glycemic regulation is blunted by curcumin and is associated to modulation of gut microbiota[J].J Nutr Biochem,2019,72(1):108218.
[98]O'KEEFE S J.Diet,microorganisms and their metabolites,and colon cancer[J].Nat Rev Gastroenterol Hepatol,2016,13(12):691-706.
[99]FERNANDES M F,DE OLIVEIRA S,PORTOVEDO M,et al.Effect of short chain fatty acids on age-related disorders[J].Adv Exp Med Biol,2020,1260(1):85-105.
[100]LI C W,ZHAO Y,CHENG J,et al.A proresolving peptide nanotherapy for site-specific treatment of inflammatory bowel disease by regulating proinflammatory microenvironment and gut microbiota[J].Adv Sci(Weinh),2019,6(18):1900610.