近几十年来,糖尿病一直是困扰人类的难题。它是一类以高血糖为临床特征的代谢疾病。高血糖主要是因为胰岛素分泌不足或胰岛素无法发挥其生物学功能。长期的高血糖可能引发各种组织的病变,尤其是眼、肾、心脏等。糖尿病分为1 型和2 型,我国2 型糖尿病(Type 2 diabetes mellitus,T2DM)占据总糖尿病人群的90%左右。T2DM 主要表现为胰岛素抵抗 (Insulin resistance,IR),即机体不能对胰岛素产生正常反应的状态。
胰岛素抵抗是一种病理状态,即依赖胰岛素的组织(如肝脏、骨骼肌和脂肪)无法对正常循环水平的胰岛素做出合适的反应。IR 与T2DM、高血压、肥胖等疾病可能同时存在,且具有一定的因果关系。1988年美国科学家Reaven 将这一类代谢性疾病统称为“代谢综合征X”[1]。1995年Stern 创建了 “共同土壤理论”,IR 是此类代谢性疾病发生、发展的共因[2]。2010—2016年流行病学调查发现,全球每年因糖尿病致死的大约有160 万人,死亡率达2.67 人/万人,已成为第七大致死疾病[3]。2018年我国患代谢综合征达33.9%(男性31.0%,女性36.8%),且逐年递增,严重影响人们的正常工作和生活[4]。
目前,胰岛素抵抗的致病机制还存在一定的争议。越来越多的证据表明,胰岛素抵抗与高脂膳食、肠道菌群和胆汁酸的代谢存在密切的联系。本文从高脂膳食、肠道菌群和胆汁酸代谢3 个方面分别讨论与胰岛素抵抗之间的关系。
1 胰岛素抵抗
1.1 胰岛素抵抗的信号通路及机制
胰岛素是一种由胰岛β 细胞分泌的51 个氨基酸组成的内源性生物活性肽,它与靶细胞质膜上的胰岛素受体(Insulin receptor,INSR)特异性结合以产生生物学功能[5]。INSR 是一种由α2β2 结构的四聚体组成的跨膜糖蛋白,每个亚基都含有1个酪氨酸激酶结构域。INSR 有A 和B 亚型,B 亚型对胰岛素更具特异性。B 亚型主要在分化的肝脏、肌肉和脂肪组织中表达,它介导胰岛素的大部分代谢效应[6]。INSR 有2 个胰岛素结合位点,互相表现出负相关调控,即当一个位点与胰岛素结合时,另一位点的亲和力下降。1 个胰岛素分子只能激活1 个INSR[7]。
INSR 主要通过有丝分裂信号和代谢信号这两种方式激活下游的信号通路。有丝分裂信号主要涉及受体酪氨酸激酶共有的丝裂原活化蛋白激酶通路的激活。活化的INSR 首先通过招募磷酸化酪氨酸结合支架蛋白来刺激下游信号,进而激活下游效应器[8]。INSR 通过结合几种磷酸化酪氨酸结合蛋白递送下游信号,如参与有丝分裂通路生长因子受体结合蛋白2 (Growth factor receptorbound protein,GRB2)和同源区结构域蛋白C(Homology domain containingprotein C,SHC),参与代谢信号通路的胰岛素受体底物 (Insulin receptor substrate,IRS)[9]。当IRS 的PTB(Phosphotyrosine binding)结构域与INSR 的pTyr972 结合后,INSR 磷酸化多个IRS 酪氨酸残基,进而招募下游信号效应器来传递和放大胰岛素信号[10]。而INSR 的去磷酸化是由蛋白酪氨酸磷酸酶 (Protein tyrosine phosphatase,PTPases)来实现的,尤其是PTP1B。激活后的INSR 立即通过激活NAD(P)H 氧化酶4(NOX4)来抑制PTP1B 的活性[11-12]。由此可见,PTP1B 可以通过逆转胰岛素诱导的IRS-1 酪氨酸残基磷酸化,从而削弱胰岛素信号转导[13]。
酪氨酸磷酸化的IRS 蛋白招募含有调节性p85 亚基和催化性p110 亚基的磷脂酰肌醇-3-激酶(Phosphatidylinositol 3-kinase,PI3K)异二聚体。各种PI3K 亚基敲除模型的相关研究表明,PI3K是胰岛素信号传导中必不可少的一环[8,14-15]。若消除PI3K 的活性,则抑制了胰岛素的信号转导相关生物学功能。PI3K 催化磷脂酰肌醇-4,5-二磷酸(Phosphatidylinositol-4,5-bisphosphate,PIP2)合成磷脂酰肌醇-3,4,5-三磷酸 (PIP3),PIP3 将具有PH(pleckstrin homology)结构域的蛋白招募到质膜上,帮助共同定位下游的信号效应器——磷脂酰肌醇依赖激酶1 和AKT(Protein kinase B)。活化的AKT 在不同的功能途径中磷酸化许多下游底物,使其成为胰岛素信号分支中的关键节点。AKT 共有3 种亚型,AKT1、AKT2 和AKT3,其中AKT2 在胰岛素的代谢途径中起到重要作用,它们主要通过促进骨骼肌和脂肪细胞中葡萄糖转运蛋白(如GLUT-4)的易位,进而增加葡萄糖的摄取[16]。
1.2 胰岛素抵抗的发病机制
胰岛素信号转导非常复杂,这个过程有多种酶和调节蛋白的参与,它们的表达或功能障碍均可影响胰岛素的正常生理作用。虽然IR 的确切病理生理学尚不明确,但是胰岛素信号转导障碍是最核心因素,具体有以下几个诱因:
1) 脂质 甘油三酯不会直接损害细胞胰岛素敏感性,尚不明确哪种脂质成分与胰岛素抵抗有联系。目前研究关注的的重点包括以下3 种脂质成分:二酰甘油(Diacylglycerol,DAG)、神经酰胺和酰基肉碱,它们与肝脏和骨骼肌胰岛素抵抗的发病机制有关。已有强有力的证据表明DAG/nPKC 轴的激活在脂质诱导的胰岛素抵抗中起因果作用,而且野生型啮齿动物和人的相关性研究中也提示了它们生理学上的相关性[17]。Summers等[18]研究表明神经酰胺诱导骨骼肌中胰岛素抵抗的作用机制最为明晰,而其在肝脏和白色脂肪组织(White adipose tissue,WAT)中的机制尚未明确。在某些模型中神经酰胺信号可直接诱发胰岛素抵抗,而脂质诱导的胰岛素抵抗与神经酰胺信号无关[17]。酰基肉碱是脂肪酸β 氧化未完全的一种产物,胰岛素抵抗个体肌肉组织中往往具有高浓度的酰基肉碱。研究表明酰基肉碱可能是通过损害胰岛素诱导的AKT 磷酸化信号和增加活性氧的浓度来诱导机体胰岛素抵抗[19]。
2) 炎症因子 炎症因子是胰岛素抵抗的关键因子之一。研究表明肥胖诱导的促炎细胞因子与IR 密切相关。Toll 样受体(Toll-like receptors,TLR)激活是诱发肥胖相关炎症的重要因素,尤其是TLR-2 和TLR-4[20]。TLR 是免疫系统的一种受体,通常由病原体相关分子(如脂多糖)激活,再通过核因子κB(Nuclear factor kappa-B,NF-κB)途径诱导炎症。而在IR 个体中,大量促炎细胞因子和炎症介质上调,特别是肿瘤坏死因子-α(Tumor necrosis factor-α,TNF-α)、单核细胞趋化蛋白-1(Monocyte chemotactic protein 1,MCP-1)、C-反应蛋白 (C-reactive protein,CRP) 和白细胞介素(Interleukin,IL)。它们主要通过:①激活IKKβ/NF-κB 和JNK 通路,IRS-1 第307 位丝氨酸磷酸化,降低GLUT-4 的表达;②激活细胞外信号调节激酶1/2 (Extracellular signal-regulated kinases 1 and 2,ERK1/2),从而降低IRS-1 的表达;③依赖于SOCS1 和SOCS3 的机制诱导IRS1 的降解。
3) 脂肪因子 脂肪因子是一类由脂肪细胞产生和释放的具有特殊生物活性的分子,包括脂联素和瘦素等。这些脂肪因子参与糖脂代谢调控,与肥胖和胰岛素抵抗的发生和发展密切相关。低脂联素血症是由基因或者导致肥胖的环境因素相互作用引起的,它在胰岛素抵抗的发生中具有重要作用[21]。脂联素可通过与其受体AdipoR1 和AdipoR2 结合,激活AMPK 通路,增加乙酰辅酶A羧化酶的磷酸化以减少糖异生,促使脂肪酸分解及葡萄糖转运,进而调控机体糖代谢[22]。另一个重要的脂肪因子是瘦素,瘦素在肥胖个体中水平升高,皮下脂肪含量是决定机体瘦素水平的主要因素。瘦素信号通过Janus 激酶、信号转导和转录激活因子(JAK-STAT)途径传递[23],它的主要生理作用是抑制食欲,刺激产热,增强脂肪酸氧化,降低血糖,减轻体重和脂肪累积[23]。研究表明,肥胖伴随胰岛素抵抗的个体中往往也表现出一定程度的瘦素抵抗[24]。
4) 内质网应激和线粒体功能障碍 内质网是真核细胞中的一个重要细胞器,参与伴侣蛋白和糖苷酶对胰岛素等蛋白质的合成、折叠、包装和运输[25]。高血糖、氧化应激和药物等许多刺激可破坏内质网的平衡状态,进而诱导内质网应激[25]。内质网应激造成蛋白质折叠中断,导致错误折叠蛋白质的积累,并激活未折叠蛋白质反应,进而促进炎症发生和脂质堆积,并对胰岛素信号、胰岛素生物合成和胰岛β 细胞功能产生负面影响。
线粒体是主要的代谢细胞器,在组织依赖型的胰岛素信号传递中起着重要作用[26]。胰岛素对线粒体的正常功能至关重要,它通过抑制FOXO11/HMOX12 和维持线粒体中NAD+/NADH 的比率来维持线粒体电子传输链的完整性[26]。自由基诱导的氧化应激在IR 中起着重要作用,而自由基主要来自于线粒体[27-28]。虽然胰岛素的敏感性可被依赖线粒体的自由基激活,但是过量的自由基会减弱胰岛素信号并导致胰岛素抵抗[26]。胰岛素和线粒体之间是互相依存关系,线粒体融合离不开胰岛素,胰岛素信号转导也离不开线粒体[29]。
2 高脂膳食、肠道菌群与胰岛素抵抗
2.1 膳食对肠道菌群的影响
膳食是调节肠道菌群组成的一个关键因素,长期的膳食习惯对人体肠道菌群有很大影响[30]。例如,与肠杆菌科(主要是志贺氏菌和大肠杆菌)水平较高的意大利儿童相比,食用大量植物多糖的非洲儿童的粪便肠道菌群中厚壁菌门水平较低,拟杆菌门水平较高(主要是普雷沃菌和木聚糖原小单胞菌)[31]。人体肠道菌群可分为3 个不同的肠型,每种肠型都由不同的属主导,如拟杆菌、普雷沃菌和瘤胃球菌。以Bacteroides[2]为主的肠型与食用富含蛋白质和动物脂肪的膳食有关,普雷沃菌为主的肠型与富含碳水化合物的膳食有关[32]。瘤胃球菌肠型与拟杆菌肠型难以区分,它们有部分共有的特征菌。从非洲农村儿童肠道菌群与膳食的相关性研究中发现,普雷沃菌与高碳水化合物膳食高度相关。然而,短期的膳食干预(10 d)不足以改变个体的肠型,需要长期的膳食改变才能显著改变肠道菌群的组成[33]。
每日碳水化合物摄入量的变化可能会在短时间内影响特定的结肠菌群。膳食添加益生元菊粉可以增加人体粪便中普氏栖粪杆菌和双歧杆菌的水平[34]。同样的,在膳食诱导的肥胖小鼠中,益生元可以选择性增加双歧杆菌属的丰度,而这与脂肪生成和LPS 水平的降低有关[35]。膳食中添加抗性淀粉可以增加粪便中与纤维素发酵相关菌布氏瘤胃球菌和直肠真杆菌的水平,而不同宿主的肠道微生物对抗性淀粉的作用也并不相同,这表明膳食干预需有针对性[36]。
肠道菌群对膳食脂肪也有很大的响应。喂食高脂饲料的小鼠拟杆菌门的数量减少,厚壁菌门和变形菌门的数量增加[37-38]。这种变化是迅速的,发生在24 h 内[39]。与移植普通小鼠供体的菌群相比,将高脂膳食诱导肥胖小鼠的盲肠菌群移植到无菌受体小鼠,显著增加了受体的肥胖率[38]。肥胖小鼠菌群的改变在促进膳食诱导的肥胖方面可能起到一定的作用,而导致这一现象的机制尚不清楚。膳食的改变明显改变了肠道菌群,这可能对宿主的代谢表型有一定的贡献。
2.2 肠道菌群的功能
肠道菌群(Gut microbiota)是指其宿主肠道内微生物群落,包括细菌、病毒、真菌、古生菌和噬菌体等。人体肠道菌群在宿主的生理活动中起着关键作用,它是一个由数万亿个细菌组成的“器官”,这些细菌大多居住在结肠末端。人体中的肠道菌质量约为1.5 kg,它携带的基因至少是人类宿主的150 倍[40]。人类基因组计划研究表明人体内主要的菌群为拟杆菌门(Bacteroidetes)、厚壁菌门(Firmicutes)、变形菌门(Proteobacteria)、放线菌门(Actinobacteria)和疣微菌门(Verrucomicrobia)[41]。在属水平上,菌的分类和功能更为复杂多样,如表1所示。肠道菌的功能主要为:①参与食品成分的代谢过程;②调控肠道屏障;③调节免疫[42]。
表1 肠道菌群分类及其特点
Table 1 Classfication and character of gut microbes

功能或特点文献厚壁菌门(Firmicutes)瘤胃球菌属(Ruminococcus)分解纤维素和抗性淀粉,降解黏蛋白,产丁酸[59],[60]梭菌属(Clostridium)致病菌(产毒素):C.perfringens;C.difficile益生菌(产丁酸):C.butyricum门属[61],[62]乳杆菌属(Lactobacillus)一些菌株为益生菌;富含胆盐水解酶;可以利用单糖、双糖等;产乙酸、黏液结合蛋白等真杆菌属(Eubacterium)含胆盐水解酶和转运酶,利用非消化碳水化合物,产丁酸[42],[63][64],[65]粪杆菌属(Faecalibacterium)产丁酸[64]罗氏菌属(Roseburia)产丁酸[64]拟杆菌门(Bacteriodetes) 拟杆菌属(Bacteroides)富含糖基水解酶,利用聚糖、己糖胺和唾液酸,产丙酸[66]普雷沃菌属(Prevotella)降解纤维素和木聚糖,产丙酸[42],[64]木聚糖原小单胞菌属(Xylanibacter)降解纤维素和木聚糖[42],[64]放线菌门(Actinobacteria) 柯林斯菌属(Collinsella)与系统炎症正相关,水解胆汁酸[67]双歧杆菌属(Bifidobacterium)益生菌;富含胆汁外排转运体,胆盐水解酶和ATP 酶;能分解植物多糖、低聚糖和黏蛋白;产细菌素、乳酸和乙酸等[63],[68]放线菌门(Proteobacteria) 埃希氏菌属(Escherichia)病原菌,产脂多糖(Lipopolysaccharide,LPS)和乙醇[69]脱硫弧菌属(Desulfovibrio)还原硫酸盐,促炎[70]疣微菌门(Verrucomicrobia)阿克曼菌属(Akkermansia)降解黏蛋白,产丙酸[64]古生菌(Euryachaeota)甲烷短杆菌属(Methanobrevibacter)降解多糖,产甲烷[71]
肠道微生物的膳食依赖性作用主要为发酵多糖,调控胆汁酸和胆碱代谢。非消化性碳水化合物是肠道菌群的重要能量来源,多形拟杆菌和卵形拟杆菌等含有的糖苷酶和裂解酶基因的数量是人类基因组的两倍多,并且能够利用几乎所有的主要植物和宿主多糖(如黏液相关糖蛋白),产生短链脂肪酸和单糖调控宿主的能量代谢[43-44]。肠道菌群还可以通过胆汁酸代谢途径生成宿主所需的营养成分,如维生素等[42]。此外,胆碱对肝脏的脂质代谢和极低密度脂蛋白的合成也很重要,膳食中胆碱含量不足会诱发肠道微生态的改变和肝脏脂肪变性[45-46]。
肠道菌群定植于肠道中,与肠黏膜共同组成肠道的生物屏障,促进肠细胞增殖和分泌黏蛋白与连接蛋白,以保持肠道屏障功能的完整性[47]。肠道菌群紊乱时,有害微生物(如:大肠杆菌和艰难梭菌)会显著增加,进而降低肠上皮细胞连接蛋白的表达,破坏肠道屏障,增加血浆中内毒素含量,加剧宿主的系统性炎症及肠道炎症等相关疾病的发生[48-49]。相反,肠道益生菌的代谢物丁酸可以通过低氧诱导因子-1 (Hypoxia inducible factor-1,HIF-1)改善肠上皮细胞的屏障功能[4],HIF-1 在维持肠屏障完整性中发挥着关键作用[50]。此外,丁酸可通过诱导编码紧密连接成分的基因和激活其它转录因子(STAT3 和SP1)进行蛋白质重组来促进上皮屏障功能[51]。丁酸是人结肠细胞获得能量的主要来源,可进行肠道糖异生,对血糖和能量平衡有良好的作用[52]。丁酸是肠上皮细胞通过β 氧化消耗大量氧气所必需的,它会产生一种维持肠道氧平衡的缺氧状态,从而防止菌群失调[53]。
肠道菌群在肠道和全身免疫系统的调节中起着重要作用,对于肠道相关淋巴组织的成熟、免疫球蛋白A(Immunoglobulin A,IgA)的分泌和重要抗菌肽的产生等是必不可少的[54]。肠道菌群紊乱会立即导致细菌抗原移位增加,并极大地改变宿主免疫反应,引起慢性炎症和代谢功能障碍[55]。同样的,宿主免疫系统也可以通过释放β 防御素、隐蔽素、凝集素、血管生成素4、活性氧、IgA 和细菌素等分子来调控菌群结构,从而减少各种病原菌的生长[56-57]。
虽然小鼠与人体的肠道菌群仅有15%的共有菌属,但是小鼠模型仍然是研究肠道菌群相关疾病潜在机制的有力工具[58]。对比常见的与肠道菌群相关的疾病发现,在小鼠与人体中肥胖和肠易激综合征相关的肠道菌群的主要变化是一致的[58]。
2.3 高脂膳食条件下肠道菌群影响宿主胰岛素敏感性及其潜在机制
能量摄入过多和运动减少,是与代谢性疾病发展密切有关的两个传统环境因素。在这些最常见的诱因中,高脂膳食是导致代谢紊乱的主要因素。而这些代谢疾病的发生不能完全归因于人类基因组、营养习惯的变化,肠道菌群在这里也发挥着重要的作用[72]。Rabot 等[73]研究发现无菌C57BL/6J 小鼠不会发生高脂膳食诱导的胰岛素抵抗。紊乱的肠道菌群可能会诱导机体产生胰岛素抵抗[74]。由此可见,肠道菌群在高脂膳食诱导的胰岛素抵抗的发生和发展中起着至关重要的作用。本文从以下几个方面讨论肠道菌群在胰岛素抵抗中发挥作用的潜在机制(图1)。
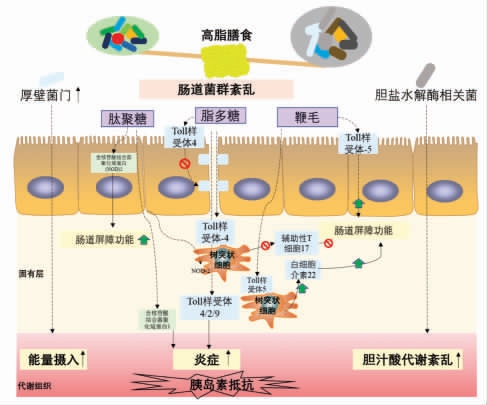
图1 肠道菌群与胰岛素抵抗
Fig.1 Gut microbiota and insulin resistance
1) 能量代谢 通过研究肥胖和代谢综合征的肠道菌群,发现肥胖患者肠道菌群的组成和代谢功能发生了重大变化[75],这可能与肥胖正相关的肠道菌(“胖菌”)能够从膳食中提取更多能量有关[76]。此外,这些研究表明,菌群可与宿主上皮细胞相互作用,间接控制能量消耗和储存[77]。肠道菌群促进能量代谢的功能很可能归因于厚壁菌门的丰度增加,它具有代谢不溶性碳水化合物的能力,从而获得更高的能量[78]。然而,它们之间作用的确切机制在很大程度上仍未可知。
2) 炎症 高脂膳食可以诱导富含脂多糖(LPS) 的肠道菌富集,从而导致血浆LPS 浓度升高,这是代谢性内毒素血症的一大特征[79]。HFD 通过LPS 诱导Toll 样受体4(TLR-4)通路激活,从而减少紧密连接蛋白,使得肠道通透性变强,肠黏膜屏障受损[79-80]。益生元干预可以通过调节微生物驱动的肠L 细胞分泌内源性的胰高血糖素样肽2(Glucagon-like peptide 2,GLP-2) 来逆转这种损害[81]。HFD 还降低了黏液层厚度,在服用产黏液的嗜黏蛋白阿克曼菌 (Akkermansia muciniphila)或益生元后,黏液层厚度恢复到正常状态,这主要是通过内源性大麻素系统恢复嗜黏蛋白阿克曼菌丰度来调节肠道屏障功能的[82]。此外,肠道菌群可以通过干扰抗原呈递细胞诱导调节性T17(Th17)细胞。HFD 是通过改变肠道菌群促进肠道Th17 细胞的丢失,从而降低肠道的防御性和完整性[83]。将Th17 细胞移植到肥胖小鼠体内,可增加拟杆菌门与厚壁菌门的比例和阿克曼菌的丰度,使肠道菌群的构型与瘦的个体相似,从而改善受体的代谢功能[84]。
细菌易位的机制主要与微生物相关的分子模式的宿主识别受体结合,包括与细菌LPS 结合的CD14 和肽聚糖(Peptidoglycan,PG)传感器NOD1[85]。然而,在高脂膳食诱导的代谢疾病中,PG 通过NOD2 改善肠道屏障和膳食诱导的组织炎症[86-87]。因此,调控特定肠道菌的黏附和定植可能成为预防或逆转代谢紊乱的有效策略。如益生菌治疗的策略,包括双歧杆菌和阿克曼菌,可通过调节菌群结构来有效地防止黏膜细菌的黏附和移位、组织炎症和胰岛素抵抗[85,88-89]。同样的,在膳食诱导的代谢综合征模型中,将卡姆果多酚作为益生元摄入,也可以发现阿克曼菌的丰度增加并发挥类似的保护作用[90]。胞壁酰二肽(微生物细胞壁的一种成分)在代谢性内毒素血症、血糖和肥胖状态下,通过NOD2 的途径来降低炎症并促进胰岛素信号转导的作用[91]。
当LPS 转运到代谢组织后,通过TLR4 介导的途径,并在TLR2 和TLR9 的参与下,诱导促炎反应,从而在肥胖和2 型糖尿病状态下抑制胰岛素信号转导[45,92-93]。研究表明代谢性内毒素血症诱导的表型在抗生素治疗后随着炎症标志物和肠道通透性的降低而减弱,这表明代谢性内毒素血症是由肠道菌群介导的[79]。此外,5-氨基水杨酸等抗炎剂在代谢综合征模型中能有效地重塑肠道屏障,减轻菌群紊乱和炎症[94]。肠道菌群动态平衡也受到肠道炎症小体的调节,炎症小体的缺失可诱导菌群失调并加剧代谢性内毒素血症,从而引起非酒精性脂肪肝,包括肝脏脂肪变性、系统炎症和胰岛素抵抗[45]。
代谢性内毒素血症还会诱导内质网应激,并激活组蛋白乙酰转移酶p300,阻止其与胰岛素受体的联系,损害胰岛素信号[95]。同样,依赖于TLR5的信号通路,通过识别细菌鞭毛来调节肠道微生态,提供对代谢性疾病的保护[96-97]。髓样细胞凋亡初级反应88 (Myeloid differentiation primary response 88,MyD88) 是TLR 信号通路中的重要信号分子。值得注意的是,组织特异性MyD88 缺陷具有不同的表型。具体而言,肠道特异性MyD88的缺失可能有助于肠道屏障功能正常化,并以依赖菌群的方式增加高脂膳食的能量消耗,从而减少脂肪生成和炎症,并改善葡萄糖稳态[98]。相反,肝细胞中特异性MyD88 的缺失可能通过诱导胆汁酸信号和肠道菌群紊乱来破坏糖脂代谢的动态平衡[99]。这些研究表明MyD88 可作为代谢性疾病的药物靶点。
3) 胆汁酸代谢 随着研究的深入,胆汁酸(Bile acids,BAs) 与菌群的相互作用被认为是影响宿主代谢的关键因素之一。胆汁酸在脂质消化过程中发挥着关键作用,其代谢受肠道微生物调控。胆汁酸经由肝脏内源性生成,然后通过肠道微生物进一步代谢。胆汁酸是通过法尼醇X 受体(Farnesoid X receptor,FXR) 和G 蛋白偶联BAs受体 (Takeda G protein-coupled receptor 5,TGR5) 共同调节新陈代谢和炎症的信号分子[100]。以下详细介绍胆汁酸与肠道菌群、胰岛素抵抗的关系。
3 肠道菌群、胆汁酸与胰岛素抵抗
3.1 肠道菌群与胆汁酸代谢
胆汁酸是由胆固醇代谢产生的两亲性类固醇分子,它们可以形成胶束,促进肠道营养物质、脂质和亲脂性维生素的吸收、乳化和转运[101]。胆汁酸的形成过程是复杂的,包括至少17 种酶的催化反应[102]。胆汁酸的合成发生在肝脏,可以通过两种途径完成(表2、图2)。在正常条件下,通过经典(或中性)途径至少产生了75%的胆汁酸,它是由胆固醇7α - 羟化酶 (Cholesterol 7α -hydroxylase,CYP7A1)催化胆固醇7α-羟基化反应来启动的[103]。CYP7A1 是限速酶,它决定胆汁酸池的大小。另一种替代(或酸性)途径是由甾醇-27-羟化酶(Sterol 27α-hydroxylase,CYP27A1)启动的[104],形成的27-羟基胆固醇被氧甾醇-7α-羟化酶(Oxysterol 7βhydroxylase,CYP7B1)进一步羟化。由胆固醇和氧甾醇生成的7α-羟基化中间体,随后,在几个反应步骤中经历甾醇环修饰和侧向改变氧化和缩短[103]。Sayin 等[105]研究表明,肠道菌群可调节CYP7A1、CYP7B1 和CYP27A1 的表达。
表2 胆表汁酸的分类和代谢途径
Table 2 The classfications and metabolic ways of bile acids
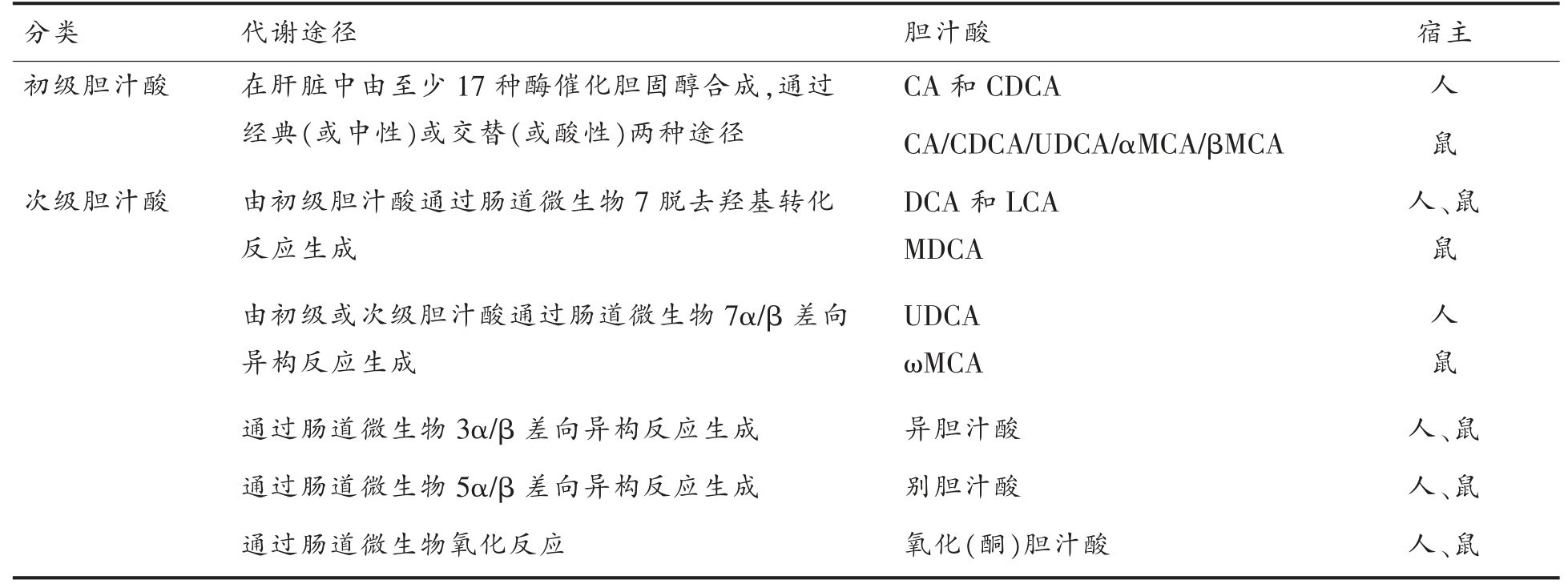
分类代谢途径胆汁酸宿主初级胆汁酸在肝脏中由至少17 种酶催化胆固醇合成,通过经典(或中性)或交替(或酸性)两种途径CA 和CDCA人CA/CDCA/UDCA/αMCA/βMCA鼠次级胆汁酸由初级胆汁酸通过肠道微生物7 脱去羟基转化反应生成DCA 和LCA MDCA人、鼠鼠由初级或次级胆汁酸通过肠道微生物7α/β 差向异构反应生成UDCA ωMCA人鼠通过肠道微生物3α/β 差向异构反应生成异胆汁酸人、鼠通过肠道微生物5α/β 差向异构反应生成别胆汁酸人、鼠通过肠道微生物氧化反应氧化(酮)胆汁酸人、鼠
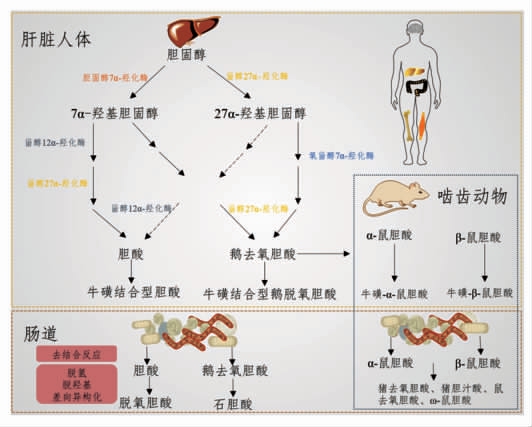
图2 胆汁酸合成与代谢[108]
Fig.2 Bile acid synthesis and metabolism[108]
经典途径同时产生胆酸 (Cholic acid,CA)和鹅去氧胆酸(Chenodeoxycholic acid,CDCA),而替代途径主要产生CDCA。这两种主要胆汁酸之间的比例由甾醇12α-羟化酶 (sterol 12α-hydroxylase,CYP8B1)决定,它是CA 合成所必需的酶,而这种酶不受微生物的调节[105-106]。除了CA 和CDCA,小鼠还产生鼠胆酸 (Muricholic acids,MCAs)和熊去氧胆酸(Ursodeoxycholic acid,UDCA)作为主要胆汁酸[105]。在人体中,UDCA 是一种次级胆汁酸,通常检测不到MCAs。在无菌或抗生素处理的小鼠或大鼠中,它们的胆汁酸池主要由初级结合胆汁酸组成[107]。无菌大鼠实验表明,MCAs 是由CDCA 在肝脏中合成的。此外,肠道菌群对肝脏合成MCAs 有很强的调节作用[105]。
肝脏中初级胆汁酸通过两步反应在C-24 位与甘氨酸或牛磺酸结合[108],该过程受肠道微生物调控[105]。然后,结合胆汁酸通过胆盐输出泵被主动输送到胆汁中,并储存在胆囊中,直到进食后释放到十二指肠。胆汁酸的两亲性结构赋予它们乳化特性,促进膳食脂质和脂溶性维生素的乳化和吸收。有趣的是,与常规饲养小鼠相比(约50%),无菌小鼠胆汁酸池中牛磺β 鼠胆酸(TβMCA)的比例增加(约80%),这些差异是否也会影响它们乳化脂质的能力尚不清楚。大约95%的胆汁酸会被肠道重吸收,主要以结合胆汁酸的形式在回肠末端由顶端钠依赖性胆汁酸转运体(Apical sodiumdependent bile acid transporter,ASBT)吸收,并通过门静脉再循环到肝脏并再次分泌。这个过程即胆汁酸的肠肝循环,在人类身上每天大约发生6次,该过程中ASBT 同样受肠道微生物调控,这表明肠道菌群不仅调控胆汁酸的合成,而且调控胆汁酸的重吸收。
微生物的去结合作用(即去除结合胆汁酸中甘氨酸或牛磺酸) 可通过调节ASBT 活性阻止小肠对胆汁酸的重吸收。胆汁酸的去结合是由具有胆盐水解酶(Bile salt hydrolase,BSH)活性的细菌进行的。宏基因组分析表明,功能性的BSH 存在于人体肠道的所有主要细菌和古生菌中,包括乳酸杆菌、双歧杆菌、梭菌和拟杆菌等[109-110]。与其它微生物生态系统相比,BSH 在肠道菌群中富集与抵抗胆汁毒性有关[109]。
3.2 胆汁酸代谢与胰岛素抵抗
近年来,越来越多的研究表明胆汁酸参与全身代谢、胰岛素抵抗、高血糖调节和能量消耗。BAs代谢异常与多种代谢性疾病密切相关,如肥胖、血脂异常、非酒精性脂肪肝等。此外,胆汁酸被证明与葡萄糖和脂肪代谢有关。因此,胰岛素抵抗时BAs 代谢情况,BAs 在糖代谢稳态中的调控机制和以胆汁酸为靶点的胰岛素抵抗的防治策略成为研究热点。
1) 胆汁酸在胰岛素抵抗状态下的变化 总胆汁酸水平升高是胰岛素抵抗和T2DM 的病理特征或发病诱因。在T2DM 中,总胆汁酸水平随着甘油三酯、IR 指数、血压和BMI 的升高而升高,这说明总BAs 含量与T2DM 有关[111-112]。研究表明,总胆汁酸浓度随膳食脂肪含量的增加而升高[113]。
在胰岛素抵抗情况下,不仅总胆汁酸水平显著变化,而且胆汁酸的组成也有很大的变化。比如,结合胆汁酸的比例在糖尿病小鼠中显著升高[114]。分析小鼠粪便中的胆汁酸成分表明,糖尿病小鼠较野生型小鼠排出更多的CA 和DCA,更少的MCA[115]。同时,抑制CA 合成可以改善葡萄糖稳态,预防膳食诱导的肥胖[116-117]。 相反,CA 水平的升高可能通过刺激胆固醇吸收而导致血脂紊乱、糖尿病和肥胖[118]。近年,在人群试验中发现的新代谢紊乱标志物——猪胆酸(Hyocholic acid,HCA),它的含量与肥胖和糖尿病的发病率呈现负相关[119]。
2) 胆汁酸在糖代谢稳态中的调控机制 BAs的信号主要通过FXR 和TGR5 这两个胆汁酸受体介导,所有的胆汁酸均可与FXR 结合,不同BAs 与它们的亲和力有很大的差别。而在这些胆汁酸中,CDCA 是FXR 最强的激动剂。FXR 几乎在所有的组织器官(肝脏、肠道、白色脂肪组织和心脏等)中均可表达,这使BAs 通过FXR 介导来调控不同的生理功能。FXR 与维甲酸X 受体形成异源二聚体,下调CYP7A1 的表达,进而抑制肝脏中胆固醇转化为BAs。FXR 介导的2 条抑制CYP7A1(胆汁酸合成)的机制包括:①在肝脏中,FXR 刺激小异二聚体伴侣 (Small heterodimer partner,SHP) 的表达,激活的SHP 下调CYP7A1的表达;②在肠道中,FXR 增加循环中的成纤维细胞生长因子19 (Fibroblast growth factor,FGF19;小鼠FGF15),再通过它下调CYP7A1 和CYP8B1的表达。而肠道FGF15/19 是通过肝脏FGF4/βKlotho 受体来抑制CYP7A1 和CYP8B1 的表达[120]。在肥胖小鼠中过度表达CYP7A1 不仅可以减轻小鼠体重,而且可以预防胰岛素抵抗、脂代谢紊乱、炎症和肝脏纤维化等疾病状态,这表明CYP7A1参与缓解肥胖伴随的代谢紊乱[121-122]。
全身性调控FXR 对糖代谢稳态会造成不同的结果,这可能与各个组织的FXR 调控糖代谢的机制不同有关。肝BA-FXR 信号调控餐后血糖主要是通过减少肝糖异生并刺激肝糖原生成[123-124]。小鼠模型研究表明,进食后,诱导BAs 分泌激活肝脏BA-FXR 信号,刺激糖原储存,并下调肝脏糖酵解和脂肪生成基因的表达,如碳水化合物反应元件结合蛋白和固醇调节元件结合蛋白1c[125-126]。类似地,小鼠研究表明,肝脏中激活FXR 下调了糖异生相关的酶的水平,如磷酸烯醇丙酮酸羧激酶和葡萄糖-6-磷酸酶[123-124]。然而,关于FXR 控制肝脏糖异生的潜在机制仍需进一步研究。有趣的是,另外一些研究表明FXR 并不是直接影响肝脏对胰岛素的敏感性,而是通过外周组织,如脂肪组织和骨骼肌[125,127]。肝脏FXR-SHP 信号受损可提高老龄小鼠的葡萄糖耐受性和脂肪酸代谢,恢复了衰老表型带来的肥胖和葡萄糖敏感性[128]。综上,肝脏FXR-SHP 信号在调控血糖和能量平衡上有着重要的作用。
肠道BA-FXR 介导FGF15/19 的表达,使其与肝FGFR4/βKlotho 结合,促进肝糖原合成,降低血糖[129]。FGF15 缺陷型小鼠主要生理表现为高血糖和肝糖原合成受损,而摄入FGF19 可以缓解这些症状,这表明FGF15/19 具有促进能量和糖代谢的作用[129]。同样,肥胖和糖尿病小鼠系统性地摄入FGF19 会改善糖尿病症状[130]。此外,近期研究表明,抑制下丘脑的FGF15 的表达可负面调节背侧迷走神经复合体的神经元活动,最终导致胰岛α细胞分泌胰高血糖素水平降低[131]。总之,这些结果表明FGF15/19 为肠道、肝脏和神经元的激活提供了一个信号网络,从而系统控制正常血糖和T2DM 个体中的血糖反应。最近的几项研究强调胃肠道中FXR 信号的另一个效应。阻断肠道FXR对血糖稳态和能量消耗有一定的有益作用。肠道中的FXR 信号改善了葡萄糖6-磷酸的吸收动力学,而这种效应在FXR 缺陷的小鼠中得到缓解[132]。单独肠道FXR 缺陷的小鼠表现为抵抗肥胖和胰岛素敏感性增加,这表明激活肠道FXR 促进了肥胖相关疾病的发展[133-134]。相反,Fang 等[135]研究表明,对肥胖的糖尿病小鼠给予肠道FXR 激动剂非那明(Fexaramine)治疗,可以减轻体重,改善IR,同时提升能量消耗。综上所述,大部分研究表明,肠道中激活的FXR 信号会导致肥胖引起的高血糖并减少能量消耗,而特异性地抑制肠道FXR信号有助于改善高血糖。
TGR5 在许多组织和器官中均有所表达,主要包括肠道、胆囊、棕色和白色脂肪组织、骨骼肌、大脑和胰腺。BAs 可与TGR5 结合,诱导cAMP 的产生,进而激活不同组织和细胞类型中的蛋白激酶A 通路[136-137]。
在肠道L 细胞中,BAs 激活TGR5 促使分泌胰高血糖素样肽1(GLP-1)。这种肽可以作用于胰岛β 细胞来调控葡萄糖刺激的胰岛素分泌[137]。肠L 细胞中的TGR5 信号诱导线粒体氧化磷酸化,ATP/ADP 比值升高,随后ATP 依赖性钾通道关闭,细胞内钙动员增强,从而促使GLP-1 分泌和调控葡萄糖代谢[138]。而FXR 在肠道L 细胞中通过抑制促胰高血糖素基因的表达,阻止GLP-1 的分泌[139]。这表明BAs 可以在肠道L 细胞中激活TGR5 和FXR,而它们对GLP-1 呈现出相反的调控功能。进食后,L 细胞中TGR5 迅速激活,而FXR 的激活需要一个相对延迟的反应,这导致它们对GLP-1 的调节作用在餐后反应表现为时间上的间隔[139]。T2DM 在胃搭桥(Roux-en-Y gastric bypass,RYGB)术后表现出正常的血糖,以及空腹和餐后的BAs 均显著增加。BAs 的水平随着FGF19、GLP-1 和PYY 升高而升高,然而,在这些患者中BAs-TGR5 与肠促胰岛素水平之间不呈因果关系[140-141]。在高脂膳食饲喂小鼠中,TGR5 的高表达会提高葡萄糖诱导的胰岛素水平;对肥胖和糖尿病小鼠喂养TGR5 激动剂后,会降低肝脏葡萄糖生成[138]。同样的,TGR5 相关基因敲除的肥胖小鼠表现为葡萄糖耐受性受损和胰岛素分泌不足,而这种效应在摄入TGR5 激动剂后消失[142-143]。
胰岛β 细胞表达TGR5 和FXR,它们通过提升细胞内钙浓度来促进葡萄糖刺激的胰岛素分泌[144-145]。胰岛α 细胞也可以表达TGR5,BAs 激活TGR5 来刺激α 细胞分泌GLP-1,从而促进β 细胞分泌胰岛素[146]。
除了调控GLP-1 外,TGR5 还可激活cAMP依赖的甲状腺激素激活酶,将非活性甲状腺素(Thyroxine,T4)转化为活性三碘甲状腺原氨酸(3,5,3' Triiodothyronine,T3),从而增加棕色脂肪细胞和骨骼肌的能量消耗[147]。与啮齿类动物实验结果一致,Patti 等[148]发现RYGB 术后的肥胖个体胆汁酸水平升高,而促甲状腺激素水平随之降低。另外一种连接BA 和能量消耗增加信号的机制是激活棕色脂肪组织产热。冷暴露可以刺激胆固醇脂蛋白在棕色脂肪组织的吸收和肝脏CYP7B1 的表达,从而刺激由替代途径分解胆固醇产胆汁酸,诱导肠道菌的变化,最终促进适应性产热反应[149]。
总之,啮齿动物和人体中FXR 和TGR5 信号在不同器官的信号表达,在调控糖代谢中发挥着至关重要的作用。大多数研究表明,激活肠道FXR会加剧高血糖,而激活TGR5 信号对高血糖和能量代谢发挥有效的调控作用。因此,激活TGR5 并抑制肠道FXR 可作为一种新的基于胆汁酸通路的方式,开发预防/治疗T2DM 的药物或食品开发的策略。
3) 胆汁酸防治胰岛素抵抗的药物 目前,胆汁酸防治2 型糖尿病的药物主要有4 种,分别为胆汁酸、胆汁酸螯合剂、FXR 激动剂和肠道TGR5激动剂。胆汁酸HCA 和DCA 通过激活FXR 和TGR5 增加胰岛素和GLP-1 的分泌,从而降低血糖[150-152]。常用糖尿病治疗药物二甲双胍也可以通过调节肠道菌群间接增加胆汁酸TUDCA 和GUDCA 水平,抑制肠道FXR,改善T2DM[153]。胆汁酸螯合剂最开始是用于治疗高胆固醇血症,后来发现在治疗T2DM 和胰岛素抵抗中也有显著疗效。胆汁酸螯合剂可以减少进入肝肠循环的胆汁酸,从而促进更多的胆固醇转化为胆汁酸,降低LDL-C,上调LDL 受体[154]。在调控糖代谢方面,胆汁酸螯合剂可能是通过改变胆汁酸池,激活肠道L 细胞TGR5 受体,刺激GLP-1 的分泌,进而达到降糖的效果[118]。FXR 激动剂半合成胆汁酸奥贝胆酸 (Obeticholic acid,OCA) 的激动活性是CDCA的30 倍,它们通过激活FXR 来达到调控血糖的作用。肠道TGR5 激动剂(INT-777)可能是通过抑制NF-κb,减少促炎因子产生,从而改善胰岛素抵抗[155]。
4 结语
随着研究的不断深入,高脂膳食、肠道菌群、胆汁酸代谢与胰岛素抵抗之间的关系也越来越明确。研究表明高脂膳食会导致肠道的微生态失衡,进而引起胆汁酸代谢的紊乱,从而诱发机体代谢组织产生胰岛素抵抗。虽然目前未有研究证实“高脂膳食-肠道菌群-胆汁酸” 轴在胰岛素抵抗的发生和发展中的因果关系,但是高脂膳食、肠道菌群与胆汁酸代谢之间的互相调控作用关系为胰岛素抵抗及T2DM 的防治提供了新思路。
[1]REAVEN G M.Role of insulin resistance in human disease[J].Diabetes,1988,37(12):1595-1607.
[2]STERN M P.Diabetes and cardiovascular disease:the‘common soil’ hypothesis[J].Diabetes,1995,44(4):369-374.
[3]World Health Organization.Global health estimates 2016:Deaths by cause,age,sex,by country and by region,2000-2016[Z].Geneva:World Health Organization,2018.
[4]胡盛寿,高润霖,刘力生,等.《中国心血管病报告2018》概要[J].中国循环杂志,2019,34(3):209-220.HU S S,GAO R L,LIU L S,et al.Summary of the 2018 peport on cardiovascular diseases in China[J].Chinese Circulation Journal,2019,34 (3):209-220.
[5]HAEUSLER R A,MCGRAW T E,ACCILI D.Biochemical and cellular properties of insulin receptor signalling[J].Nature Reviews Molecular Cell Biology,2018,19(1):31-44.
[6]BELFIORE A,MALAGUARNERA R,VELLA V,et al.Insulin receptor isoforms in physiology and disease:an updated view[J].Endocrine Reviews,2017,38(5):379-431.
[7]DE MEYTS P.The insulin receptor:a prototype for dimeric,allosteric membrane receptors?[J].Trends in Biochemical Sciences,2008,33(8):376-384.
[8]TANIGUCHI C M,EMANUELLI B,KAHN C R.Critical nodes in signalling pathways:insights into insulin action[J].Nature Reviews Molecular Cell Biology,2006,7(2):85-96.
[9]NAJJAR S M.Regulation of insulin action by CEACAM1[J].Trends in Endocrinology Metabolism,2002,13(6):240-245.
[10]HUBBARD S R.The insulin receptor:both a prototypical and atypical receptor tyrosine kinase [J].Cold Spring Harbor Perspectives in Biology,2013,5(3):a008946.
[11]MAHADEV K,MOTOSHIMA H,WU X,et al.The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction[J].Molecular Cellular Biology,2004,24(5):1844-1854.
[12]WU X,WILLIAMS K J.NOX4 pathway as a source of selective insulin resistance and responsiveness[J].Arteriosclerosis,Thrombosis,Vascular Biology,2012,32(5):1236-1245.
[13]YIP S C,SAHA S,CHERNOFF J.PTP1B:a double agent in metabolism and oncogenesis[J].Trends in Biochemical Sciences,2010,35(8):442-449.
[14]BRACHMANN S M,UEKI K,ENGELMAN J A,et al.Phosphoinositide 3 -kinase catalytic subunit deletion and regulatory subunit deletion have opposite effects on insulin sensitivity in mice[J].Molecular Cellular Biology,2005,25(5):1596-1607.
[15]FOUKAS L C,CLARET M,PEARCE W,et al.Critical role for the p110α phosphoinositide-3-OH kinase in growth and metabolic regulation[J].Nature,2006,441(7091):366-370.
[16]LATVA-RASKU A,HONKA M J,STANČÁKOVÁ A,et al.A partial loss-of-function variant in AKT2 is associated with reduced insulin-mediated glucose uptake in multiple insulin-sensitive tissues:a genotype-based callback positron emission tomography study [J].Diabetes,2018,67(2):334-342.
[17]PETERSEN M C,SHULMAN G I.Mechanisms of insulin action and insulin resistance[J].Physiological Reviews,2018,98(4):2133-2223.
[18]SUMMERS S A,GOODPASTER B H.CrossTalk proposal:Intramyocellular ceramide accumulation does modulate insulin resistance[J].The Journal of Physiology,2016,594(12):3167.
[19]AGUER C,MCCOIN C S,KNOTTS T A,et al.Acylcarnitines:potential implications for skeletal muscle insulin resistance[J].The FASEB Journal,2015,29(1):336-345.
[20]CHI G,FENG XX,RU YX,et al.TLR2/4 ligandamplified liver inflammation promotes initiation of autoimmune hepatitis due to sustained IL-6/IL-12/IL-4/IL-25 expression[J].Molecular Immunology,2018,99:171-181.
[21]KADOWAKI T,YAMAUCHI T,KUBOTA N,et al.Adiponectin and adiponectin receptors in insulin resistance,diabetes,and the metabolic syndrome[J].The Journal of Clinical Investigation,2006,116(7):1784-1792.
[22]KIM Y,PARK C W.Mechanisms of adiponectin action:implication of adiponectin receptor agonism in diabetic kidney disease[J].International Journal of Molecular Sciences,2019,20(7):1782.
[23]YADAV A,KATARIA M A,SAINI V,et al.Role of leptin and adiponectin in insulin resistance[J].Clinica Chimica Acta,2013,417:80-84.
[24]MYERS M G,COWLEY M A,MÜNZBERG H.Mechanisms of leptin action and leptin resistance[J].Annual Review of Physiology,2008,70:537-556.
[25]B?NHEGYI G,BAUMEISTER P,BENEDETTI A,et al.Endoplasmic reticulum stress[J].Annals-New York Academy of Sciences,2007,1113 (1):58.
[26]CHENG Z,TSENG Y,WHITE M F.Insulin signaling meets mitochondria in metabolism[J].Trends in Endocrinology Metabolism,2010,21 (10):589-598.
[27]RAINS J L,JAIN S K.Oxidative stress,insulin signaling,and diabetes[J].Free Radical Biology Medicine,2011,50(5):567-575.
[28]SEBASTIÁN D,HERNÁNDEZ -ALVAREZ M I,SEGALÉS J,et al.Mitofusin 2 (Mfn2) links mitochondrial and endoplasmic reticulum function with insulin signaling and is essential for normal glucose homeostasis[J].Proceedings of the National Academy of Sciences of the United States of America,2012,109(14):5523-5528.
[29]WESTERMEIER F,NAVARRO -MARQUEZ M,LÓPEZ-CRISOSTO C,et al.Defective insulin signaling and mitochondrial dynamics in diabetic car diomyopathy[J].Biochimica et Biophysica Acta-Molecular Cell Research,2015,1853(5):1113-1118.
[30]SINGH R K,CHANG H W,YAN D,et al.Influence of diet on the gut microbiome and implications for human health[J].Journal of Translational Medicine,2017,15(1):73.
[31]DE FILIPPO C,CAVALIERI D,DI PAOLA M,et al.Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa[J].Proceedings of the National Academy of Sciences of the United States of America,2010,107(33):14691-14696.
[32]COSTEA P I,HILDEBRAND F,ARUMUGAM M,et al.Enterotypes in the landscape of gut microbial community composition[J].Nature Microbiology,2018,3(1):8-16.
[33]WU G D,CHEN J,HOFFMANN C,et al.Linking long-term dietary patterns with gut microbial enterotypes[J].Science,2011,334(6052):105-108.
[34]RAMIREZ-FARIAS C,SLEZAK K,FULLER Z,et al.Effect of inulin on the human gut microbiota:stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii[J].British Journal of Nutrition,2008,101(4):541-550.
[35]CANI P D,NEYRINCK A M,FAVA F,et al.Selective increases of Bifidobacteria in gut microflora improve high -fat -diet -induced diabetes in mice through a mechanism associated with endotoxaemia[J].Diabetologia,2007,50(11):2374-2383.
[36]WALKER A W,INCE J,DUNCAN S H,et al.Dominant and diet -responsive groups of bacteria within the human colonic microbiota[J].The ISME Journal,2011,5(2):220-230.
[37]HILDEBRANDT M A,HOFFMANN C,SHERRILLMIX S A,et al.High-fat diet determines the composition of the murine gut microbiome independently of obesity[J].Gastroenterology,2009,137(5):1716-1724.
[38]TURNBAUGH P J,BÄCKHED F,FULTON L,et al.Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome[J].Cell Host & Microbe,2008,3(4):213-223.
[39]TURNBAUGH P J,RIDAURA V K,FAITH J J,et al.The effect of diet on the human gut microbiome:a metagenomic analysis in humanized gnotobiotic mice[J].Science Translational Medicine,2009,1(6):6ra14.
[40]TURNBAUGH P J,LEY R E,HAMADY M,et al.The human microbiome project[J].Nature,2007,449(7164):804-810.
[41]CONSORTIUM H M J R S.A catalog of reference genomes from the human microbiome[J].Science,2010,328(5981):994-999.
[42]TREMAROLI V,BÄCKHED F.Functional interactions between the gut microbiota and host metabolism[J].Nature,2012,489(7415):242-249.
[43]XU J,BJURSELL M K,HIMROD J,et al.A genomic view of the human-Bacteroides thetaiotaomi cron symbiosis[J].Science,2003,299(5615):2074-2076.
[44]MARTENS E C,LOWE E C,CHIANG H,et al.Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts[J].PLOS Biology,2011,9(12):e1001221.
[45]HENAO-MEJIA J,ELINAV E,JIN C,et al.Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity[J].Nature,2012,482(7384):179-185.
[46]SPENCER M D,HAMP T J,REID R W,et al.Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency[J].Gastroenterology,2011,140(3):976-986.
[47]JAKOBSSON H E,RODRÍGUEZ-PIÑEIRO A M,Schütte A,et al.The composition of the gut microbiota shapes the colon mucus barrier[J].EMBO Reports,2015,16(2):164-177.
[48]THEVARANJAN N,PUCHTA A,SCHULZ C,et al.Age-associated microbial dysbiosis promotes intestinal permeability,systemic inflammation,and macrophage dysfunction[J].Cell Host & Microbe,2017,21(4):455-466.
[49]LESLIE J L,HUANG S,OPP J S,et al.Persistence and toxin production by Clostridium difficile within human intestinal organoids result in disruption of epithelial paracellular barrier function[J].Infection Immunity,2015,83(1):138-145.
[50]KELLY C J,ZHENG L,CAMPBELL E L,et al.Crosstalk between microbiota -derived short -chain fatty acids and intestinal epithelial HIF augments tissue barrier function[J].Cell Host & Microbe,2015,17(5):662-671.
[51]PARADA VENEGAS D,DE LA FUENTE M K,LANDSKRON G,et al.Short chain fatty acids(SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases[J].Frontiers in Immunology,2019,10:277.
[52]DE VADDER F,KOVATCHEVA-DATCHARY P,GONCALVES D,et al.Microbiota -generated metabolites promote metabolic benefits via gut-brain neural circuits[J].Cell,2014,156(1/2):84-96.
[53]BYNDLOSS M X,OLSAN E E,RIVERA-CHÁVEZ F,et al.Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion[J].Science,2017,357(6351):570-575.
[54]BENGMARK S.Gut microbiota,immune development and function[J].Pharmacological Research,2013,69(1):87-113.
[55]BURCELIN R,GARIDOU L,POMIÉ C.Immunomicrobiota cross and talk:the new paradigm of metabolic diseases[J].Semin Immunol,2012,24(1):67-74.
[56]NEISH A S.Microbes in gastrointestinal health and disease[J].Gastroenterology,2009,136(1):65-80.
[57]FRANK D N,PACE N R.Gastrointestinal microbiology enters the metagenomics era[J].Current Opinion in Gastroenterology,2008,24(1):4-10.
[58]NGUYEN T L A,VIEIRA-SILVA S,LISTON A,et al.How informative is the mouse for human gut microbiota research?[J].Disease Models Mechanisms,2015,8(1):1-16.
[59]ZE X,DUNCAN S H,LOUIS P,et al.Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon[J].ISME J,2012,6(8):1535-1543.
[60]CROST E H,LE GALL G,LAVERDE-GOMEZ J A,et al.Mechanistic insights into the cross-feeding of Ruminococcus gnavus and Ruminococcus bromii on host and dietary carbohydrates[J].Frontiers in Microbiology,2018,9:2558.
[61]GOLIC N,VELJOVIC K,POPOVIC N,et al.In vitro and in vivo antagonistic activity of new probiotic culture against Clostridium difficile and Clostridium perfringens[J].BMC Microbiology,2017,17(1):108.
[62]KANAI T,MIKAMI Y,HAYASHI A.A breakthrough in probiotics:Clostridium butyricum regulates gut homeostasis and anti-inflammatory response in inflammatory bowel disease[J].Journal of Gastroenterology,2015,50(9):928-939.
[63]TURRONI F,VENTURA M,BUTTÓ L F,et al.Molecular dialogue between the human gut microbiota and the host:a Lactobacillus and Bifidobacterium perspective[J].Cellular Molecular Life Sciences,2014,71(2):183-203.
[64]Louis P,Flint H J.Formation of propionate and butyrate by the human colonic microbiota[J].Environmental Microbiology,2017,19(1):29-41.
[65]MUKHERJEE A,LORDAN C,ROSS R P,et al.Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health[J].Gut Microbes,2020,12(1):1802866.
[66]WEXLER H M.Bacteroides:the good,the bad,and the nitty-gritty [J].Clinical Microbiology Re views,2007,20(4):593-621.
[67]ASTBURY S,ATALLAH E,VIJAY A,et al.Lower gut microbiome diversity and higher abundance of proinflammatory genus Collinsella are associated with biopsy-proven nonalcoholic steatohepatitis[J].Gut Microbes,2020,11(3):569-580.
[68]MARTINEZ F A C,BALCIUNAS E M,CONVERTI A,et al.Bacteriocin production by Bifidobacterium spp.A review[J].Biotechnology Advances,2013,31(4):482-488.
[69]MIURA K,OHNISHI H.Role of gut microbiota and Toll-like receptors in nonalcoholic fatty liver disease[J].World Journal of Gastroenterology:WJG,2014,20(23):7381.
[70]SCANLAN P D,SHANAHAN F,MARCHESI J R.Culture-independent analysis of Desulfovibrios in the human distal colon of healthy,colorectal cancer and polypectomized individuals[J].FEMS Microbiology Ecology,2009,69(2):213-221.
[71]SAMUEL B S,HANSEN E E,MANCHESTER J K,et al.Genomic and metabolic adaptations of Methanobrevibacter smithii to the human gut[J].Proceedings of the National Academy of Sciences of the United States of America,2007,104(25):10643-10648.
[72]CANI P D,DELZENNE N M,AMAR J,et al.Role of gut microflora in the development of obesity and insulin resistance following high-fat diet feeding[J].Pathologie Biologie,2008,56(5):305-309.
[73]RABOT S,MEMBREZ M,BRUNEAU A,et al.Germ-free C57BL/6J mice are resistant to high-fatdiet -induced insulin resistance and have altered cholesterol metabolism[J].The FASEB Journal,2010,24(12):4948-4959.
[74]JIAO N,BAKER S S,NUGENT C A,et al.Gut microbiome may contribute to insulin resistance and systemic inflammation in obese rodents:a metaanalysis[J].Physiological Genomics,2018,50(4):244-254.
[75]TURNBAUGH P J,GORDON J I.The core gut microbiome,energy balance and obesity[J].The Journal of Physiology,2009,587(17):4153-4158.
[76]TURNBAUGH P J,LEY R E,MAHOWALD M A,et al.An obesity-associated gut microbiome with increased capacity for energy harvest[J].Nature,2006,444(7122):1027.
[77]BÄCKHED F,DING H,WANG T,et al.The gut microbiota as an environmental factor that regulates fat storage[J].Proceedings of the National Academy of Sciences of the United States of America,2004,101(44):15718-15723.
[78]JANDHYALA S M,TALUKDAR R,SUBRAMANYAM C,et al.Role of the normal gut micro biota[J].World Journal of Gastroenterology:WJG,2015,21(29):8787.
[79]CANI P D,BIBILONI R,KNAUF C,et al.Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice[J].Diabetes,2008,57(6):1470-1481.
[80]GUO S,NIGHOT M,AL -SADI R,et al.Lipopolysaccharide regulation of intestinal tight junction permeability is mediated by TLR4 signal transduction pathway activation of FAK and MyD88 [J].The Journal of Immunology,2015,195(10):4999-5010.
[81]CANI P D,POSSEMIERS S,VAN DE WIELE T,et al.Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability[J].Gut,2009,58(8):1091-1103.
[82]EVERARD A,BELZER C,GEURTS L,et al.Cross -talk between Akkermansia muciniphila and intestinal epithelium controls diet -induced obesity[J].Proceedings of the National Academy of Sciences of the United States of America,2013,110(22):9066-9071.
[83]GARIDOU L,POMI? C,KLOPP P,et al.The gut microbiota regulates intestinal CD4 T cells expressing RORγt and controls metabolic disease[J].Cell Metabolism,2015,22(1):100-112.
[84]HONG C P,PARK A,YANG B G,et al.Gutspecific delivery of T-helper 17 cells reduces obesity and insulin resistance in mice[J].Gastroenterolo gy,2017,152(8):1998-2010.
[85]AMAR J,CHABO C,WAGET A,et al.Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes:molecular mechanisms and probiotic treatment [J].EMBO Molecular Medicine,2011,3(9):559-572.
[86]BARREAU F,MADRE C,MEINZER U,et al.Nod2 regulates the host response towards microflora by modulating T cell function and epithelial permeability in mouse Peyer's patches [J].Gut,2010,59(2):207-217.
[87]DENOU E,LOLMÈDE K,GARIDOU L,et al.Defective NOD 2 peptidoglycan sensing promotes diet-induced inflammation,dysbiosis,and insulin re sistance[J].EMBO Molecular Medicine,2015,7(3):259-274.
[88]SHIN N R,LEE J C,LEE H Y,et al.An increase in the Akkermansia spp.population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice[J].Gut,2014,63(5):727-735.
[89]O'CALLAGHAN A,VAN SINDEREN D.Bifidobacteria and their role as members of the human gut microbiota[J].Frontiers in Microbiology,2016,7:925.
[90]ANHÊ F F,NACHBAR R T,VARIN T V,et al.Treatment with camu camu (Myrciaria dubia) prevents obesity by altering the gut microbiota and increasing energy expenditure in diet-induced obese mice[J].Gut,2019,68(3):453-464.
[91]CAVALLARI J F,FULLERTON M D,DUGGAN B M,et al.Muramyl dipeptide-based postbiotics mitigate obesity-induced insulin resistance via IRF4[J].Cell Metabolism,2017,25(5):1063-1074.
[92]CANI P D,AMAR J,IGLESIAS M A,et al.Metabolic endotoxemia initiates obesity and insulin resistance[J].Diabetes,2007,56(7):1761-1772.
[93]CREELY S J,MCTERNAN P G,KUSMINSKI C M,et al.Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes[J].American Journal of Physiology -Endocrinology Metabolism,2007,292(3):E740-E747.
[94]LUCK H,TSAI S,CHUNG J,et al.Regulation of obesity-related insulin resistance with gut anti-in flammatory agents[J].Cell Metabolism,2015,21(4):527-542.
[95]CAO J,PENG J,AN H,et al.Endotoxemia-mediated activation of acetyltransferase P300 impairs insulin signaling in obesity[J].Nature Communications,2017,8(1):1-12.
[96]VIJAY-KUMAR M,AITKEN J D,CARVALHO F A,et al.Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5[J].Science,2010,328(5975):228-231.
[97]CHASSAING B,LEY R E,GEWIRTZ A T.Intestinal epithelial cell toll-like receptor 5 regulates the intestinal microbiota to prevent low-grade inflammation and metabolic syndrome in mice[J].Gastroenterology,2014,147(6):1363-1377.
[98]EVERARD A,GEURTS L,CAESAR R,et al.Intestinal epithelial MyD88 is a sensor switching host metabolism towards obesity according to nutritional status[J].Nature Communications,2014,5(1):1-12.
[99]DUPARC T,PLOVIER H,MARRACHELLI V G,et al.Hepatocyte MyD88 affects bile acids,gut microbiota and metabolome contributing to regulate glucose and lipid metabolism[J].Gut,2017,66(4):620-632.
[100]CHÁVEZ-TALAVERA O,TAILLEUX A,LEFEBVRE P,et al.Bile acid control of metabolism and inflammation in obesity,type 2 diabetes,dyslipidemia,and nonalcoholic fatty liver disease[J].Gastroenterology,2017,152(7):1679-1694.
[101]SHAPIRO H,KOLODZIEJCZYK A A,HALSTUCH D,et al.Bile acids in glucose metabolism in health and disease[J].Journal of Experimental Medicine,2018,215(2):383-396.
[102]DE AGUIAR V T Q,TARLING E J,EDWARDS P A.Pleiotropic roles of bile acids in metabolism[J].Cell Metabolism,2013,17(5):657-669.
[103]THOMAS C,PELLICCIARI R,PRUZANSKI M,et al.Targeting bile-acid signalling for metabolic dis eases[J].Nature Reviews Drug Discovery,2008,7(8):678-693.
[104]RUSSELL D W.The enzymes,regulation,and genetics of bile acid synthesis[J].Annual Review of Biochemistry,2003,72(1):137-174.
[105]SAYIN S I,WAHLSTRÖM A,FELIN J,et al.Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid,a naturally occurring FXR antagonist[J].Cell Metabolism,2013,17(2):225-235.
[106]LI-HAWKINS J,G魡FVELS M,OLIN M,et al.Cholic acid mediates negative feedback regulation of bile acid synthesis in mice[J].The Journal of Clinical Investigation,2002,110(8):1191-1200.
[107]SELWYN F P,CSANAKY I L,ZHANG Y,et al.Importance of large intestine in regulating bile acids and glucagon-like peptide-1 in germ-free mice[J].Drug Metabolism Disposition,2015,43(10):1544-1556.
[108]CHIANG J Y L.Bile acid metabolism and signaling[J].Comprehensive Physiology,2013,3(3):1191-1212.
[109]JONES B V,BEGLEY M,HILL C,et al.Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome[J].Proceedings of the National Academy of Sciences of the United States of America,2016,105(36):13580-13585.
[110]RIDLON J M,KANG D J,HYLEMON P B.Bile salt biotransformations by human intestinal bacteria[J].Journal of Lipid Research,2006,47(2):241-259.
[111]PRAWITT J,CARON S,STAELS B.Bile acid metabolism and the pathogenesis of type 2 diabetes[J].Current Diabetes Reports,2011,11(3):160-166.
[112]VINCENT R P,OMAR S,GHOZLAN S,et al.Higher circulating bile acid concentrations in obese patients with type 2 diabetes[J].Annals of Clinical Biochemistry,2013,50(4):360-364.
[113]CUMMINGS J,WIGGINS H,JENKINS D,et al.Influence of diets high and low in animal fat on bowel habit,gastrointestinal transit time,fecal microflora,bile acid,and fat excretion[J].The Journal of Clinical Investigation,1978,61(4):953-963.
[114]MOORANIAN A,ZAMANI N,TAKECHI R,et al.Modulatory nano/micro effects of diabetes development on pharmacology of primary and secondary bile acids concentrations[J].Current Diabetes Reviews,2020,16(8):900-909.
[115]LI T,FRANCL J M,BOEHME S,et al.Glucose and insulin induction of bile acid synthesis mechanisms and implication in diabetes and obesity[J].Journal of Biological Chemistry,2012,287 (3):1861-1873.
[116]SLÄTIS K,G魡FVELS M,KANNISTO K,et al.Abolished synthesis of cholic acid reduces atherosclerotic development in apolipoprotein E knockout mice[J].Journal of Lipid Research,2010,51(11):3289-3298.
[117]KAUR A,PATANKAR J V,DE HAAN W,et al.Loss of Cyp8b1 improves glucose homeostasis by increasing GLP-1[J].Diabetes,2015,64(4):1168-1179.
[118]BERTAGGIA E,JENSEN K K,CASTRO-PEREZ J,et al.Cyp8b1 ablation prevents Western diet-induced weight gain and hepatic steatosis because of impaired fat absorption[J].American Journal of Physiology-Endocrinology Metabolism,2017,313 (2):E121-E133.
[119]ZHENG X,CHEN T,ZHAO A,et al.Hyocholic acid species as novel biomarkers for metabolic disorders[J].Nature Communications,2021,12(1):1-11.
[120]INAGAKI T,CHOI M,MOSCHETTA A,et al.Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis[J].Cell Metabolism,2005,2(4):217-225.
[121]LI T,OWSLEY E,MATOZEL M,et al.Transgenic expression of cholesterol 7α-hydroxylase in the liver prevents high-fat diet-induced obesity and in sulin resistance in mice[J].Hepatology,2010,52(2):678-690.
[122]LIU H,PATHAK P,BOEHME S,et al.Cholesterol 7α-hydroxylase protects the liver from inflammation and fibrosis by maintaining cholesterol homeostasis[J].Journal of Lipid Research,2016,57(10):1831-1844.
[123]ZHANG Y,LEE F Y,BARRERA G,et al.Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice [J].Proceedings of the National Academy of Sciences of the United States of America,2006,103 (4):1006-1011.
[124]POTTHOFF M J,BONEY-MONTOYA J,CHOI M,et al.FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1α pathway [J].Cell Metabolism,2011,13(6):729-738.
[125]MA K,SAHA P K,CHAN L,et al.Farnesoid X receptor is essential for normal glucose homeostasis[J].The Journal of Clinical Investigation,2006,116(4):1102-1109.
[126]DURAN -SANDOVAL D,CARIOU B,PERCEVAULT F,et al.The farnesoid X receptor modulates hepatic carbohydrate metabolism during the fasting-refeeding transition[J].Journal of Biological Chemistry,2005,280(33):29971-29979.
[127]CARIOU B,VAN HARMELEN K,DURAN-SANDOVAL D,et al.The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice[J].Journal of Biological Chemistry,2006,281(16):11039-11049.
[128]KIM K H,CHOI S,ZHOU Y,et al.Hepatic FXR/SHP axis modulates systemic glucose and fatty acid homeostasis in aged mice[J].Hepatology,2017,66(2):498-509.
[129]KIR S,BEDDOW S A,SAMUEL V T,et al.FGF19 as a postprandial,insulin-independent activator of hepatic protein and glycogen synthesis[J].Science,2011,331(6024):1621-1624.
[130]FU L,JOHN L M,ADAMS S H,et al.Fibroblast growth factor 19 increases metabolic rate and re verses dietary and leptin -deficient diabetes [J].Endocrinology,2004,145(6):2594-2603.
[131]PICARD A,SOYER J,BERNEY X,et al.A genetic screen identifies hypothalamic Fgf15 as a regulator of glucagon secretion[J].Cell Reports,2016,17(7):1795-1806.
[132]VAN DIJK T H,GREFHORST A,OOSTERVEER M H,et al.An increased flux through the glucose 6-phosphate pool in enterocytes delays glucose absorption in Fxr-/- mice[J].Journal of Biological Chemistry,2009,284(16):10315-10323.
[133]LI F,JIANG C,KRAUSZ K W,et al.Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity [J].Nature Communications,2013,4(1):1-10.
[134]JIANG C,XIE C,LV Y,et al.Intestine-selective farnesoid X receptor inhibition improves obesity-re lated metabolic dysfunction[J].Nature Communica tions,2015,6(1):1-18.
[135]FANG S,SUH J M,REILLY S M,et al.Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance[J].Nature Medicine,2015,21(2):159-165.
[136]KAWAMATA Y,FUJII R,HOSOYA M,et al.AG protein-coupled receptor responsive to bile acids[J].Journal of Biological Chemistry,2003,278 (11):9435-9440.
[137]KATSUMA S,HIRASAWA A,TSUJIMOTO G.Bile acids promote glucagon -like peptide -1 secretion through TGR5 in a murine enteroendocrine cell line STC-1[J].Biochemical Biophysical Research Communications,2005,329(1):386-390.
[138]THOMAS C,GIOIELLO A,NORIEGA L,et al.TGR5-mediated bile acid sensing controls glucose homeostasis[J].Cell Metabolism,2009,10(3):167-177.
[139]TRABELSI M S,DAOUDI M,PRAWITT J,et al.Farnesoid X receptor inhibits glucagon-like peptide-1 production by enteroendocrine L cells[J].Nature Communications,2015,6(1):1-13.
[140]DUTIA R,EMBREY M,O'BRIEN S,et al.Temporal changes in bile acid levels and 12α-hydroxylation after Roux-en-Y gastric bypass surgery in type 2 diabetes[J].International Journal of Obesity,2015,39(5):806-813.
[141]SACHDEV S,WANG Q,BILLINGTON C,et al.FGF 19 and bile acids increase following Roux-en-Y gastric bypass but not after medical management in patients with type 2 diabetes[J].Obesity Surgery,2016,26(5):957-965.
[142]PELLICCIARI R,GIOIELLO A,MACCHIARULO A,et al.Discovery of 6α-ethyl-23 (S)-methylcholic acid (S-EMCA,INT-777) as a potent and selective agonist for the TGR5 receptor,a novel target for diabesity [J].Journal of Medicinal Chem istry,2009,52(24):7958-7961.
[143]BRIERE D A,RUAN X,CHENG C C,et al.Novel small molecule agonist of TGR5 possesses anti-diabetic effects but causes gallbladder filling in mice[J].PLoS one,2015,10(8):e0136873.
[144]KUMAR D P,RAJAGOPAL S,MAHAVADI S,et al.Activation of transmembrane bile acid receptor TGR5 stimulates insulin secretion in pancreatic β cells[J].Biochemical Biophysical Research Communications,2012,427(3):600-605.
[145]RENGA B,MENCARELLI A,VAVASSORI P,et al.The bile acid sensor FXR regulates insulin transcription and secretion[J].Biochimica et Biophysica Acta -Molecular Basis of Disease,2010,1802(3):363-372.
[146]KUMAR D P,Asgharpour A,Mirshahi F,et al.Activation of transmembrane bile acid receptor TGR5 modulates pancreatic islet α cells to promote glucose homeostasis[J].Journal of Biological Chemistry,2016,291(13):6626-6640.
[147]WATANABE M,HOUTEN S M,MATAKI C,et al.Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation[J].Na ture,2006,439(7075):484-489.
[148]PATTI M E,HOUTEN S M,BIANCO A C,et al.Serum bile acids are higher in humans with prior gastric bypass:potential contribution to improved glucose and lipid metabolism[J].Obesity,2009,17(9):1671-1677.
[149]WORTHMANN A,JOHN C,RÜHLEMANN M C,et al.Cold-induced conversion of cholesterol to bile acids in mice shapes the gut microbiome and promotes adaptive thermogenesis[J].Nature Medicine,2017,23(7):839.
[150]RYAN K K,TREMAROLI V,CLEMMENSEN C,et al.FXR is a molecular target for the effects of vertical sleeve gastrectomy[J].Nature,2014,509(7499):183-188.
[151]KOHLI R,BRADLEY D,SETCHELL K D,et al.Weight loss induced by Roux-en-Y gastric bypass but not laparoscopic adjustable gastric banding increases circulating bile acids [J].The Journal of Clin ical Endocrinology Metabolism,2013,98(4):E708-E712.
[152]ZHENG X,CHEN T,JIANG R,et al.Hyocholic acid species improve glucose homeostasis through a distinct TGR5 and FXR signaling mechanism[J].Cell Metabolism,2021,33(4):791-803.
[153]SUN L,XIE C,WANG G,et al.Gut microbiota and intestinal FXR mediate the clinical benefits of metformin[J].Nature Medicine,2018,24(12):1919-1929.
[154]CORSINI A,WINDIER E,FARNIER M.Colesevelam hydrochloride:usefulness of a specifically engineered bile acid sequestrant for lowering LDL -cholesterol[J].European Journal of Cardiovascular Prevention Rehabilitation,2009,16(1):1-9.
[155]POLS T W,NOMURA M,HARACH T,et al.TGR5 activation inhibits atherosclerosis by reducing macrophage inflammation and lipid loading[J].Cell Metabolism,2011,14(6):747-757.