抑郁症(Depression)是最常见的精神疾病,以情绪低落、兴趣减退、睡眠和饮食紊乱以及意志活动减退为主要特征,全球有2.6 亿人深受其害[1]。抑郁症状长期持续且容易反复发作,它严重影响个人的工作、学习和日常生活,给个人和社会带来巨大的负担[2]。抑郁症患者还有较高的自杀行为倾向,导致死亡和伤残。随着现代生活节奏的加快,社会压力增大,其发病率近年来呈上升趋势。我国约有5 000 万抑郁人口,占总人口的4.2%,其中每年有近900 万人因抑郁伤残[1],抑郁症是我国致残的第二大原因[3],已成为全社会广泛关注的问题。
抑郁症的发病机制相当复杂,遗传因素以及童年时期经历都与抑郁风险密切相关,生活中的创伤、压力等环境因素都有可能诱发抑郁症[4]。与高发病率不匹配的是低治疗率,仅有不到50%的抑郁患者接受治疗,且疗效较差[5]。目前许多化学合成抗抑郁药的疗效都不甚完美,存在反应延迟[6],甚至有严重的副作用[7]。越来越多的研究人员将目标对准天然活性物质,尤其是药食同源的物质,以寻求高效、安全、低毒的抗抑郁物质。
本文汇总近年来国内外学者的相关研究,结合抑郁产生的机制,对具有潜在抗抑郁效果的活性物质进行综述,总结一些具有较强效果的物质及其作用机制,并提出建议。这对今后筛选新型、安全有效的抗抑郁活性成分具有重要意义,并可为新型缓解抑郁产品的开发提供参考。
1 抗抑郁机制
抑郁症产生的病理机制非常复杂,尽管通过不懈研究已取得了较大进展,但是单一的机制很难解释疾病的成因,不同机制可能是异质性的基础。现今广为接受的理论主要有单胺假说、海马-垂体-肾上腺轴理论、神经可塑性、炎症刺激四大理论,通过不同方面影响抑郁症。
1.1 单胺假说
单胺类神经递质是一类重要的兴奋性神经递质,包括五羟色胺(5-HT)、去甲肾上腺素(NE)和多巴胺(DA)等,单胺假说认为抑郁背后的机制是一种或几种单胺类神经递质水平异常。20 世纪中期发现降压药利血平可通过耗竭脑内单胺类神经递质浓度引发抑郁症[8],这引起了人们对单胺在抑郁中的作用的研究兴趣。同样,色氨酸(5-HT 合成所必需的一种氨基酸)的耗竭也被证明会在治愈患者中再次诱发抑郁症状[9]。许多早期抗抑郁药的开发都基于单胺耗竭理论,如单胺氧化酶抑制剂(MAOIs)、三环类抗抑郁剂(TCAs)、选择性5-羟色胺再摄取抑制剂(SSRIs)等,都可以提高突触间隙内单胺神经递质浓度,改善抑郁症状[10]。
尽管此类抗抑郁药在几小时内就能改善单胺浓度,却无法解释为何改善抑郁症状需要数天甚至数周,为何临床应用时对部分人群无效[11]。研究也证明将SSRIs 抗抑郁药与其它治疗联用(如认知行为疗法)的效果会更好[12],反映了这一假设的不足之处。尽管如此,单胺神经递质及其受体活性仍可作为抑郁的指标,并在不同脑区起不同的作用。
1.2 海马-垂体-肾上腺(HPA)轴
下丘脑-垂体-肾上腺(HPA)轴是一个反馈回路,在这个回路中,压力激活激素通路,诱导机体对感知到的威胁做出“战斗或逃跑”的反应。当遇到压力时,下丘脑分泌促肾上腺皮质激素释放激素(CRH),之后CRH 刺激脑垂体分泌促肾上腺皮质激素(ACTH),接着ACTH 刺激肾上腺皮质释放肾上腺皮质激素(CORT)[13]。皮质激素的升高会提高神经系统的兴奋性,使机体保持亢进状态。应激过后,CORT 通过负反馈调节降低CRH 浓度,使整个HPA 轴恢复正常。尽管皮质激素的升高有利于机体应对突发状况,但长期暴露于压力之下会导致不益影响[14],一旦反馈通路失灵,或压力长期存在,会导致HPA 轴持续亢进,边缘系统受到过度刺激,进而产生抑郁样行为[15]。有超过40%的抑郁症患者有皮质醇增多或不同程度的HPA 系统紊乱[16]。小鼠转基因过表达CRF 会导致抑郁行为,而脑内注射CRF 拮抗剂改善了这种症状[17],同样证明HPA 轴负反馈失效是产生抑郁的原因之一。因此,HPA 系统的激素变化也常作为指标,来探究抗抑郁症的机制。
大脑中广泛分布的糖皮质激素受体(GR)和盐皮质激素受体(MR)可与皮质激素结合,有效的负反馈调节HPA 轴活性[16]。然而直接调节受体活性的药物临床抗抑郁效果不佳[4]。已有研究发现HPA 轴与免疫的关系,尽管皮质激素对免疫反应起双重作用,但抑郁患者高皮质醇确实伴随着高免疫因子的状况[18]。DNA 甲基化可能是HPA 轴负反馈调节与免疫功能之间联系的机制,HPA 轴负反馈钝化可导致皮质醇水平的长期增加,进而导致肿瘤坏死因子(TNF)甲基化降低和TNF 信号通路的增强,NF-κB 信号可能是HPA 轴与免疫功能之间的交互作用的中介[19]。
1.3 神经可塑性
神经元水平上的生长和适应性被更广泛地称为神经可塑性,包括神经细胞的生成与凋亡,新突触的激活与抑制。大脑具有很强的可塑性,能够快速创建和消除突触,并在适应和学习过程中改变连接回路[20]。抑郁患者在这种细胞水平上的症状主要包括海马新生细胞减少,神经元凋亡,海马缩小等[21]。因此,神经元可塑性和神经元损伤被认为与抑郁症的病理密切相关,针对这方面的治疗能改善抑郁症。
神经可塑性的变化受神经营养因子的调节。神经营养因子是大脑中重要的一类信号分子,负责轴突定位、神经元生长、发育过程中突触的成熟和突触可塑性。这一蛋白家族包括神经生长因子(NGF)、脑源性神经营养因子(BDNF)等。其中,BDNF 在突触可塑性及许多精神疾病的病理或治疗中均发挥重要作用[22]。研究发现,BDNF 减少使压力致抑郁的易感性增加[23],诊断为抑郁症的患者血清BDNF 水平降低,抑郁症患者的BDNF 可以通过抗抑郁治疗恢复[24]。大鼠在BDNF 敲低后可诱发抑郁行为[25],脑内缺乏BDNF 会影响抗抑郁药物的疗效[26],因此可以将BDNF 作为反映抑郁症的重要指标和抑郁症治疗的主要靶点。
神经可塑性可以与炎症和HPA 轴相互作用,炎症及HPA 轴的改变最终都引起神经可塑性的变化[4]。皮质酮长期升高可以降低整个海马区BDNF 蛋白水平[27];BDNF 调节5-HT 受体和转运体的功能,减少5-HT 转运,与SSRI 一样增加了神经递质浓度[28-29]。
1.4 炎症刺激
越来越多的研究数据支持炎症与抑郁症的联系,抑郁症患者表现出脑内和外周炎症因子浓度的升高,小胶质细胞激活[30]。患有自身免疫性疾病和严重感染的人更容易患抑郁症,用干扰素(IFN)和白细胞介素(Interleukin)等细胞因子进行治疗会引发抑郁症[31-32]。Meta 分析得出结论,外周血中IL-1β、IL-6、TNF-α 和C 反应蛋白(CRP)是抑郁症患者中可靠的炎症标记[33-34],同时也是抑郁治疗的靶点,针对炎症因子的治疗可以改善抑郁[35]。
动物实验也证明,免疫刺激可以激活小胶质细胞等免疫细胞,直接产生大量炎症因子。小鼠注射脂多糖(LPS)可以诱发炎症反应,致使海马内TNF-α、IL-1β 升高,并诱导抑郁样行为[36]。面对压力时,核转录因子κB(NF-κB)可以被激活,促进炎症因子前体,如pro-IL-1β 的产生;炎症因子前体的切割成熟需要炎症小体(如NOD 样受体蛋白3 NLRP3)参与[37]。阻断NLRP3 炎症小体可以改善应激诱导的小鼠外周血和大脑中IL-1β 的增加,同时消除小鼠的抑郁样行为[38]。不止脑内免疫反应,脑外炎症因子可以在穿过血脑屏障后直接作用,或者由传入通路(如迷走神经)传导信号于神经元和胶质细胞[39]。此外,免疫刺激也会通过引起HPA 轴的过度活跃、单胺递质代谢紊乱和神经营养问题等症状,导致抑郁的发生。
2 抗抑郁活性物质
许多现存抗抑郁药物都存在治疗周期长,治疗率低的问题,一种药物可能只对部分患者起到有限的作用,对60%~70%的患者没有缓解作用,对30%~40%的患者没有显著反应[6],同时副作用较大[40],并且停药易复发[18]。为了避免不必要的不良反应,越来越多的科学研究聚焦一些食药同源成分,包括维生素、黄酮类、皂苷类等成分,探究它们的抗抑郁效果及作用机制。
天然物质取材广泛,更贴近饮食,副作用小,具有多靶点作用的特点。前述抑郁机制的四大理论在抑郁症中相互关联(图1),每个系统内部的变化都会影响其它系统,系统之间的相互关联性是天然物质多靶点有效性的基础。如今天然活性物质已变成疾病预防、治疗的研究热点,结合近些年的研究,综述了抗抑郁活性成分的研究进展(表1)。
表1 药食同源抗抑郁活性成分及涉及机制
Table 1 Mechanisms of anti-depression components homologous with medicine and food
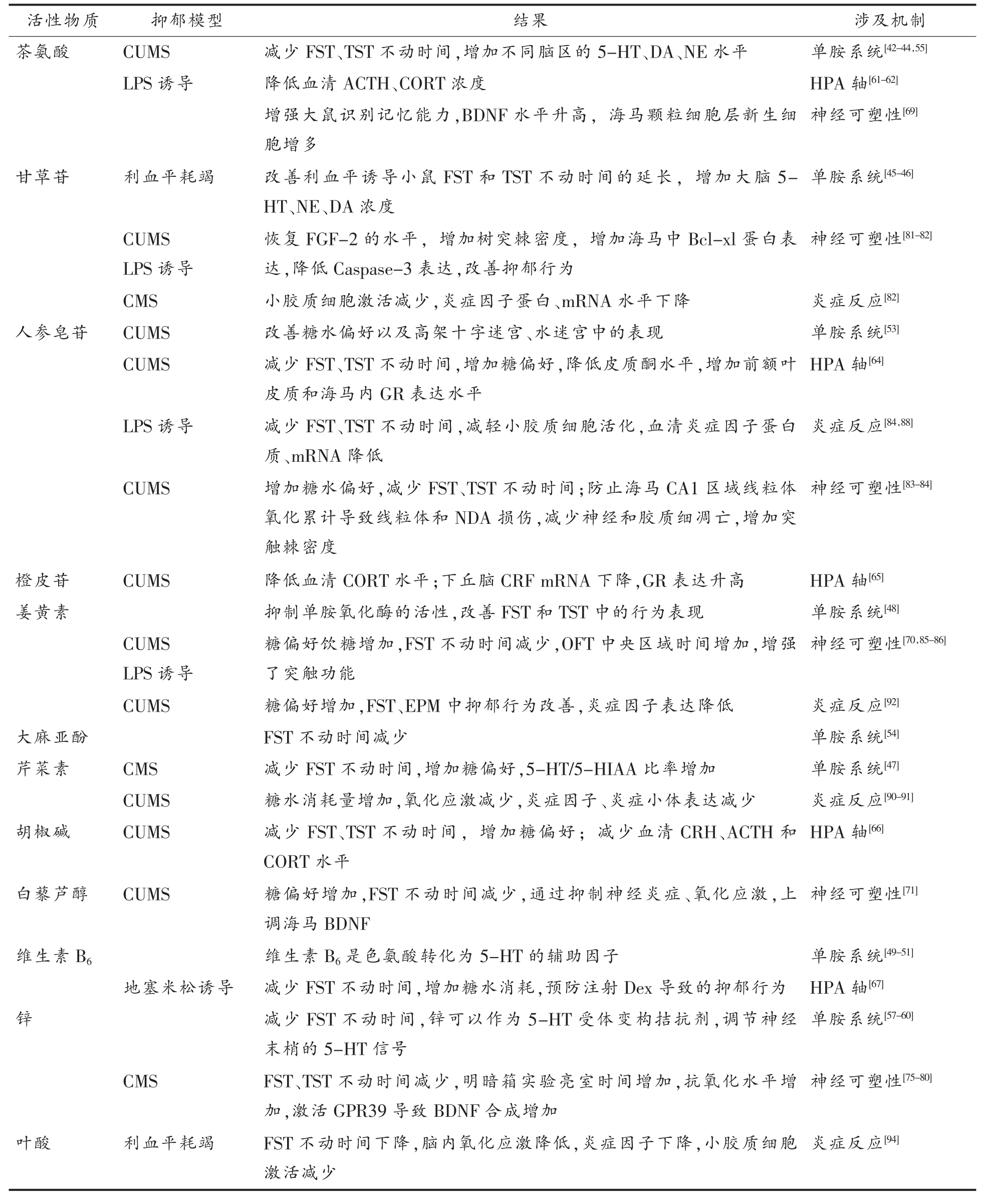
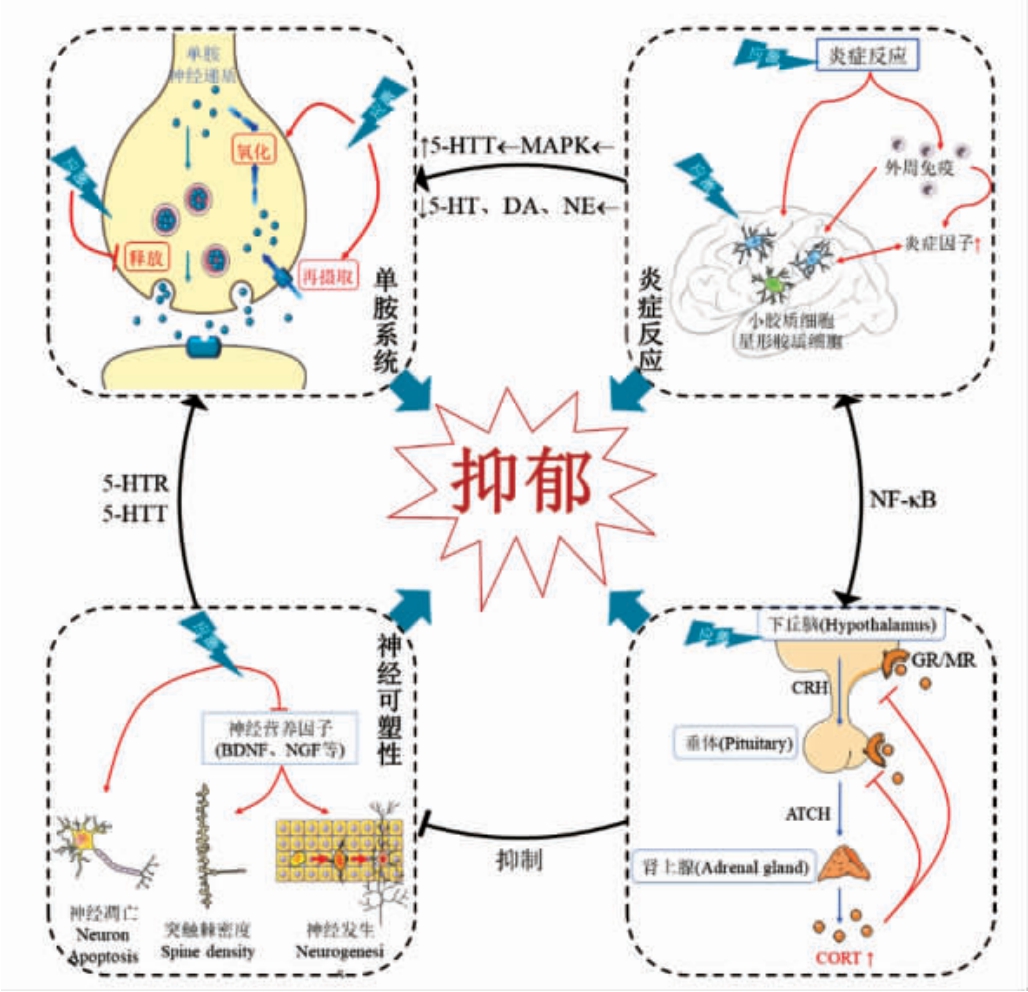
图1 抑郁症产生的四大机制
Fig.1 The four main mechanisms of depression
2.1 调节单胺系统
单胺系统是抗抑郁最早的针对目标,大量研究发现天然活性成分可以提高单胺神经递质浓度。比如:茶,一向被认为具有调节情绪的作用,研究证实茶叶中的L-茶氨酸具有抗抑郁作用[41]。Yin等[42]采用强迫游泳实验(FST)、悬尾实验(TST)、旷场实验和利血平实验,证明L-茶氨酸的抗抑郁效果与单胺神经递质系统相关。长期灌胃给予茶氨酸可以显著增加大鼠慢性不可预测应激(CUMS)模型各脑区的多巴胺(DA)、五羟色胺(5-HT)、去甲肾上腺素(NE)等单胺神经递质水平,同时降低了谷氨酸水平,改善抑郁症[43]。最近的一项研究测定了茶氨酸对CUMS 模型中不同脑区递质水平的影响,除海马内只有DA 增加外,其余各脑区、各种单胺递质均有所上升[44]。甘草苷、异甘草苷作为甘草内的主要黄酮类活性成分,均可通过增加海马、下丘脑和皮质中5-HT 和NE 水平,以显著缩短小鼠FST 和TST 的静止时间[45]。甘草提取物减少了利血平诱导小鼠FST 和TST 的不动时间,通过增加大脑NE 和DA,实现抗抑郁作用[46]。洋甘菊提取物中的主要活性成分——芹菜素也能影响单胺递质系统,改善慢性轻度应激(CMS)诱导的糖偏好减少,减少了小鼠FST 的静止时间,改善了不同脑区5-HT 及其代谢物5-羟基吲哚乙酸(5-HIAA)、多巴胺(DA)的变化[47]。
进一步研究证实,许多天然活性成分与单胺氧化酶抑制剂类似,通过抑制单胺氧化酶的活性,减少神经递质被氧化,达到增加突触间隙内递质浓度的目的。姜黄素作为一种食品着色剂,能抑制小鼠大脑中单胺氧化酶的活性,提高5-HT、NE 和DA 浓度,改善FST 和TST 中的行为表现[48]。如维生素B6 是色氨酸转化为5-HT 的辅助因子,在单胺神经递质代谢过程中必不可少,是单胺氧化酶正常生产所必需的微量元素[49],人群研究表明,维生素B6 的摄入量与抑郁症风险呈显著负相关[50],低血浆维生素B6 的衍生物——磷酸吡哆醛(PLP)与抑郁症状相关[51],补充维生素B6 可以缓解年长妇女的抑郁症状[52]。
除此之外,调节单胺递质受体功能也能对恢复单胺系统起作用。人参皂苷Rb1 通过升高5-HT1A 受体水平,改善了CUMS 大鼠在糖水偏好以及高架十字迷宫、水迷宫中的表现[53]。大麻二酚(CBD),抗抑郁效果被5-HT1A 受体拮抗剂WAY100635 阻断,表明CBD 的作用是通过激活5-HT1A 受体实现的[54]。茶氨酸使海马内DA 增加,此作用被多巴胺D1/5 受体拮抗剂阻断,说明茶氨酸激活D1/5 受体-PKA 通路,改善了海马单胺递质状况[55]。锌作为人体必需微量元素之一,是体内许多酶和其它蛋白的组成部分[56]。锌在FST中的抗抑郁作用被5-HT 合成抑制剂、5-HT 受体拮抗剂阻断,表明锌的抗抑郁作用可能涉及5-羟色胺系统[57]。进一步研究发现,锌可以作为5-HT1A、5-HT7 受体变构拮抗剂,调节神经末梢的5-HT 信号[58-59]。锌离子对5-HT 受体的作用具有两面性,低浓度时作为激动剂,高浓度时起拮抗剂作用[60]。这些研究表明,神经递质受体是很好的干预靶点,对抑郁症的作用比较强烈。然而仍待深入研究,发现更多可作用于受体的天然活性物质和更精确的作用机理。
药食同源的天然活性物质调节单胺系统的主要方式包括:抑制单胺氧化酶,提高单胺递质浓度,调节单胺递质受体功能。然而,还有很多具体机制可以提高单胺递质浓度,如促进神经递质释放,抑制递质再摄取等,活性物质的作用是否涉及这些方面还需要更多研究。同时,某些物质可能不止涉及1 种调节单胺系统的方式,因此需要更多、更全面、更系统的实验来验证。
2.2 调节下丘脑-垂体-肾上腺(HPA)轴
研究表明,天然活性物质可以调节HPA 轴来缓解抑郁症,它们通过降低CORT,增加糖皮质激素受体(GR)表达,调节HPA 轴的负反馈,抑制HPA 轴亢进,促进HPA 轴正常化实现抗抑郁。
茶氨酸可以穿过血脑屏障调节糖皮质激素受体(GR),减轻脂多糖(LPS)诱导的ACTH 和CORT 升高[61]。Wang 等[62]进行类似的实验,也发现了L-茶氨酸对HPA 轴的影响,认为茶氨酸可能参与了炎症因子与HPA 轴之间的相互作用。脑内的L-茶氨酸可以减少谷氨酸从前突触到突触间隙的释放,增加抑制性神经递质GABA 浓度[63],进而抑制使HPA 轴亢进。人参皂苷Rg1 也是通过增加海马和前额叶皮层中GR 表达,调节负反馈改善HPA 轴,降低皮质酮水平,起到抗抑郁作用[64]。橙皮苷是从柑桔类果皮中提取的天然黄酮类物质,研究发现橙皮苷显著改善了CUMS 大鼠过高的血清CORT 水平,并能抑制下丘脑CRF mRNA表达,上调室旁核GR 蛋白表达,抑制HPA 轴亢进[65]。
除调节GR 功能外,调节压力回路上游的CRH、ACTH 等激素也是缓解抑郁症的潜在靶点。胡椒碱属桂皮酞胺类生物碱,主要存在于胡椒科中,Hu 等[66]灌胃给予CUMS 大鼠胡椒碱21 d 后,发现胡椒碱可以通过减少血清ACTH 和CRF 水平,显著改善抑郁导致的行为障碍。地塞米松是一种人工合成的皮质类固醇,体内注射可以模拟皮质醇升高和HPA 轴紊乱,预先注射维生素B6 可以预防由地塞米松引起的抑郁,减少小鼠FST 不动时间,增加糖水消耗[67]。
很多活性物质可以通过调节HPA 轴发挥抗抑郁作用,但仍需要更多的研究来寻找确切的机制,从而开发出更有针对性的治疗抑郁症的新方法和新成分。有学者认为,将抑郁症的亚型与其神经内分泌变化联系起来可能会有帮助[68],直接抑制HPA 轴相关激素分泌可能效果并不理想[16]。
2.3 改善神经可塑性
神经可塑性是近几年抑郁症的研究重点,天然活性物质可以重建神经元的可塑性,缓解神经损伤,在抑郁症治疗中发挥作用。
茶氨酸对情绪的调节具有多种作用机制,除作用于单胺系统和HPA 轴以外,它还可以优化大脑关键区域的BDNF 水平,促进神经元健康。长期服用茶氨酸后,大鼠的海马BDNF 水平显著升高,海马颗粒细胞层新生细胞显著增加[69]。姜黄素可以增加CUMS 模型中pCREB/CREB 比值与BDNF、突触后致密蛋白-95(PSD-95),改善抑郁导致的树突棘密度和总树突长度的降低[70]。白藜芦醇则是通过发挥抗炎、抗氧化作用,提高CUMS 模型大鼠BDNF 水平,起到抗抑郁作用[71]。锌存在于大脑的皮层、海马体和杏仁核等区域的突触囊泡内,随时释放调节大脑锌稳态[72]。饮食中缺乏锌会影响大脑中的锌稳态,导致精神障碍[73-74]。补锌可以增加大脑皮层BDNF,诱导神经发生[75-76],锌可以激活pERK-BNDF 通路增加大鼠大脑BDNF 的表达[77]。锌在CMS 模型中增加了皮层和海马BNDF的表达,改善了抑郁状态[78]。进一步研究表明BDNF 的增加可能与Zn2+敏感型G 蛋白偶联受体(GPR39)的激活有关[79]。锌激活GPR39 导致cAMP响应元件结合蛋白(CREB)表达增加,诱导BDNF的合成,进而激活TrkB 受体[80],刺激一系列促进神经发生的相关通路。
除增加神经营养因子外,许多物质通过抑制神经细胞凋亡、促进神经生长发育起抗抑郁作用。从甘草中提取的总黄酮(甘草苷、甘草素等),灌胃干预治疗CUMS 大鼠,提高了大鼠海马中Bcl-xl蛋白,降低Caspase-3,表明其通过抗凋亡、保护神经细胞来改善抑郁行为[81]。甘草苷在新研究中将神经可塑性与免疫联系起来,可以恢复LPS 诱导的抑郁小鼠FGF-2 的水平,增加树突棘密度,减少海马小胶质细胞激活并降低炎症因子水平,改善抑郁行为[82]。人参皂苷Rg1 也可以抑制CUMS诱导的神经元凋亡,增加Bcl-2 的表达,降低前额叶皮质(PFC)区域内Caspase-3 和Caspase-9 的表达,抑制p-38 MAPK、NF-κB 的激活[83]。Rg1 还能抑制CUMS 大鼠海马CA1 区域氧化活性,防止线粒体氧化累计导致的线粒体和NDA 损伤,减少神经和胶质细胞凋亡[84]。姜黄素对IL-1β 诱导的神经元凋亡具有保护作用[85],在LPS 诱导的大鼠抑郁模型中也证明了这一点,姜黄素通过抑制氧化应激和神经元凋亡,防止了海马CA1 区突触过度缺失,增强了突触功能,发挥神经保护作用[86]。随着技术的进步,突触可塑性的研究越来越深入并逐渐成为主流,为未来的精神疾病研究指引方向。然而在神经营养因子信号的区域特异性方面仍需做大量工作,其在不同脑区、不同作用的区别与联系仍待阐明。进一步阐明突触可塑性机制能更好地理解触发天然活性物质作用的因素,以期开发出更好的产品。
2.4 减轻炎症
许多研究发现活性物质可以通过调节炎症因子和免疫系统来改善抑郁症。通过抑制脑内免疫细胞,降低炎症因子浓度,防止炎症反应对神经系统的伤害。
人参皂苷作为人参中一类天然产物,具有良好的免疫调节作用[87]。人参皂苷Rg3 处理可有效改善LPS 诱导的抑郁模型中体质量下降、厌食、TST 与FST 不动时间增加,减轻小胶质细胞活化和NF-κB 通路激活,降低血清IL-6、TNF-α,并促进色氨酸代谢恢复平衡[88]。人参皂苷Rg1 也能够降低海马炎症反应水平,抑制小胶质细胞数目的增加,降低炎症因子IL-1β、IFN-γ、TNF-α 的mRNA 水平,最终改善抑郁行为[84]。甘草苷同样可以减少海马小胶质细胞激活,减少IL-1β、IL-6、TNF-α mRNA 表达[82]。
芹菜素广泛存在于柑橘类水果中,具有抗炎和抗氧化等多种生物作用[89]。在氧化应激的作用下,CUMS 大鼠PFC 中NLRP3 炎症小体被激活,IL-1β 分泌增加。芹菜素可上调过氧化物酶体增殖物激活受体(PPARγ)的表达,来抑制IL-1β 的产生和NLRP3 炎症小体的表达[90]。在原代小胶质细胞中,芹菜素能拮抗干扰素诱导的小胶质细胞STAT1 的磷酸化和CD40 表达,减轻其激活,抑制TNF-α、IL-6 分泌[91]。姜黄素同样能有效缓解CUMS 模型鼠的抑郁样状态,抑制NF-κB 的激活,降低促炎细胞因子(IL-1β、IL-6、TNF-α)mRNA 的表达,并抑制应激诱导的NLRP3 炎症小体的激活,从而减少IL-1β 向成熟IL-1β 的转化[92]。B 族维生素叶酸,在所有年龄段的中枢神经系统功能中都有重要作用,叶酸缺乏可能会导致抑郁、痴呆、胎儿畸形等多种中枢神经问题[93],可以通过抗氧化、减轻炎症方面缓解抑郁。灌胃叶酸显著降低利血平抑郁模型大鼠在FST 中的不动时间,通过降低脑内脂质过氧化(LPO)水平,提高超氧化物歧化酶(SOD)、谷胱甘肽(GSH)、过氧化氢酶(CAT)水平,降低了脑内IL-1β、TNF-α 水平,减少了小胶质细胞的激活[94]。
尽管通过调节免疫缓解抑郁已取得较大进展,但是仍有许多问题有待解决。多种细胞因子被活性物质下调,然而这些细胞因子在抑郁症中的具体作用尚不明确。仅针对脑内免疫(小胶质细胞)一个靶点,没有考虑外周免疫对中枢系统的影响,不够全面。过度抑制炎症会产生免疫抑制,而饮食调节更安全,更符合生理健康。随着相关研究的增加,炎症在抑郁症中的作用将变得更清晰。
3 总结
抑郁症的机制复杂,涉及多个系统、多个蛋白质和许多小分子。目前人们对其产生的机制还未解析透彻,主流的抑郁症学说:单胺假说、海马-垂体-肾上腺轴理论、神经可塑性、免疫刺激理论对抗抑郁研究仍然具有重要的指导作用。没有单一的理论或信号通路能够完全解释抑郁机制[95],因此,应该考虑各种因素,注意不同机制之间的相互作用,只有这样才能全面系统地阐述抑郁症的发病机制。
抑郁症的预防比治疗更重要[96],由于天然活性物质具有高效、安全、低毒的特点,适用于长效预防。这些天然活性物质的作用是值得肯定的,但要明确这些有效成分的关键作用机制,并建立统一的疗效诊断和评价标准。这就需要开展更深入的研究,寻找其确切的机制,从而开发出更有针对性的新方法和物质。本文总结了不同天然活性物质的抗抑郁机制,可以为开发新型抗抑郁配方,预防和治疗抑郁症提供参考。
[1] WHO.Depression and other common mental disorders:Global health estimates[EB/OL].(2021-03-31)[2021-10-11].https://www.who.int/news-room/factsheets/detail/depression.
[2] 江开达.精神病学[M].北京:北京人民卫生出版社,2005:123-124 JIANG K D.Psychiatry[M].Beijing:People's Medical Publishing House,2005:123-124
[3] ZHOU M,WANG H,ZENG X Y,et al.Mortality,morbidity,and risk factors in China and its provinces,1990-2017:A systematic analysis for the Global Burden of Disease Study 2017 [J].The Lancet,2019,394(10204):1145-1158.
[4] MALHI G S,MANN J J.Depression[J].The Lancet.2018,392(10161):2299‐2312.
[5] 彭代辉,方贻儒.我国抑郁障碍的临床与发病机制研究现状[J].上海交通大学学报(医学版),2010,30(6):609-611.PENG D H,FANG Y R.Clinical and pathogenesis researches on depressive disorder in China[J].Journal of Shanghai Jiaotong University(Medical Science),2010,30(6):609-611.
[6] TRIVEDI M H,RUSH A J,WISNIEWSKI S R,et al.Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D:Implications for clinical practice[J].American Journal of Psychiatry,2006,163(1):28-40.
[7] WILLIAMS J W,MULROW C D,CHIQUETTE E,et al.A systematic review of newer pharmacotherapies for depression in adults:Evidence report summary[J].Annals of Internal Medicine,2000,132(9):743-756.
[8] BUNNEY W E,DAVIS J M.Norepinephrine in depressive reactions.A review[J].Arch Gen Psychiatry,1965,13(6):483-494.
[9] BELL C.Tryptophan depletion and its implications for psychiatry[J].Br J Psychiatry,2001,178(5):399-405.
[10] HIRSCHFELD R.History and evolution of the monoamine hypothesis of depression[J].The Journal of Clinical Psychiatry,2000,61(suppl 6):4-6.
[11] HARMER C J,DUMAN R S,COWEN P J.How do antidepressants work? New perspectives for refining future treatment approaches[J].The Lancet Psychiatry,2017,4(5):409-418.
[12] CLEVENGER S S,MALHOTRA D,DANG J,et al.The role of selective serotonin reuptake inhibitors in preventing relapse of major depressive disorder[J].Therapeutic Advances in Psychopharmacology,2018,8(1):49.
[13] ROTHENBERG D O,ZHANG L.Mechanisms underlying the anti-depressive effects of regular tea consumption[J].Nutrients,2019,11(6):1361.
[14] RUSSELL G,LIGHTMAN S.The human stress response[J].Nature Reviews Endocrinology,2019,15(9):525-534.
[15] STETLER C,MILLER G E.Depression and hypothalamic-pituitary-adrenal activation:A quantitative summary of four decades of research[J].Psychosomatic Medicine,2011,73(2):114.
[16] KELLER J,GOMEZ R G,WILLIAMS G H,et al.HPA axis in major depression:Cortisol,clinical symptomatology and genetic variation predict cognition[J].Molecular Psychiatry,2017,22(4):527-536.
[17] STENZELPOORE M P,HEINRICHS S C,RIVES S,et al.Overproduction of corticotropin-releasing factor in transgenic mice:A genetic model of anxiogenic behavior[J].Journal of Neuroscience the Official Journal of the Society for Neuroscience,1994,14(1):2579-2584.
[18] IOB E,KIRSCHBAUM C,STEPTOE A.Persistent depressive symptoms,HPA-axis hyperactivity,and inflammation:The role of cognitive -affective and somatic symptoms[J].Molecular Psychiatry,2020,25(5):1130-1140.
[19] PALMA-GUDIEL H,PRATHER A A,LIN J,et al.HPA axis regulation and epigenetic programming of immune-related genes in chronically stressed and non-stressed mid-life women[J].Brain Behavior and Immunity,2021,92(2):49-56.
[20] FERRARI F,VILLA R F.The neurobiology of depression:An integrated overview from biological theories to clinical evidence[J].Molecular Neurobiology,2017,54(7):4847-4865.
[21] SHELINE Y I.Neuroimaging studies of mood disorder effects on the brain[J].Biological Psychiatry,2003,54(3):338-352.
[22] LEE B H,KIM H,PARK S H,et al.Decreased plasma BDNF level in depressive patients[J].J Affect Disord,2007,101(1/2/3):239-244.
[23] DUMAN C H,SCHLESINGER L,KODAMA M,et al.A Role for MAP kinase signaling in behavioral models of depression and antidepressant treatment[J].Biological Psychiatry,2007,61(5):661-670.
[24] MOLENDIJK M L,SPINHOVEN P,POLAK M,et al.Serum BDNF concentrations as peripheral manifestations of depression:Evidence from a systematic review and meta-analyses on 179 associations(N=9484)[J].Molecular Psychiatry,2014,19(7):791-800.
[25] TALIAZ D,STALL N M,DAR D,et al.Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis[J].Molecular Psychiatry,2010,15(1):80-92.
[26] ADACHI M,BARROT M,AUTRY A E,et al.Selective loss of brain-derived neurotrophic factor in the dentate gyrus attenuates antidepressant efficacy[J].Biological Psychiatry,2008,63(7):642-649.
[27] JACOBSEN J,MRK A.Chronic corticosterone decreases brain-derived neurotrophic factor(BDNF)mRNA and protein in the hippocampus,but not in the frontal cortex,of the rat-ScienceDirect[J].Brain Research,2006,1110(1):221-225.
[28] HENSLER J G,ADVANI T,MONTEGGIA L M.Regulation of serotonin-1A receptor function in inducible brain-derived neurotrophic factor knockout mice after administration of corticosterone[J].Biological Psychiatry,2007,62(5):521-529.
[29] MÖSSNER R,DANIEL S,ALBERT D,et al.Serotonin transporter function is modulated by brainderived neurotrophic factor(BDNF)but not nerve growth factor(NGF)[J].Neurochemistry International,2000,36(3):197-202.
[30] SETIAWAN E,WILSON A A,MIZRAHI R,et al.Role of translocator protein density,a marker of neuroinflammation,in the brain during major depressive episodes[J].Jama Psychiatry,2015,72(3):268-275.
[31] RAISON C L,CAPURON L,MILLER A H.Cytokines sing the blues:Inflammation and the pathogenesis of depression [J].Trends in Immunology,2006,27(1):24-31.
[32] HARRISON N A,BRYDON L,WALKER C,et al.Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity[J].Biological psychiatry,2009,66(5):407-414.
[33] MILLER A H,MALETIC V,RAISON C L,et al.Inflammation and Its Discontents:The role of cytokines in the pathophysiology of major depression[J].Biological Psychiatry,2009,65(9):732-741.
[34] PASCO J A,NICHOLSON G C,WILLIAMS L J,et al.Association of high-sensitivity C-reactive protein with de novo major depression[J].Br J Psychiatry,2010,197(5):372-377.
[35] RAISON C L,RUTHERFORD R E,WOOLWINE B J,et al.A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression:The role of baseline inflammatory biomarkers[J].Jama Psychiatry,2013,70(1):31-41.
[36] O'CONNOR J C,LAWSON M A,ANDRÉ C,et al.Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice[J].Molecular Psychiatry,2009,14(5):511-522.
[37] SWANSON K V,DENG M,TING P Y.The NLRP3 inflammasome:Molecular activation and regulation to therapeutics[J].Nature Reviews Immunology,2019,19(8):477-489.
[38] ZHANG Y,LIU L,LIU Y Z,et al.NLRP3 inflammasome mediates chronic mild stress -induced depression in mice via neuroinflammation[J].International Journal of Neuropsychopharmacology,2015,18(8):pyv006.
[39] MILLER A H,RAISON C L.The role of inflammation in depression:From evolutionary imperative to modern treatment target[J].Nature Reviews Immunology,2016,16(1):22-34.
[40] HANSEN R A,GARTLEHNER G,CAREY T S,et al.Efficacy and safety of second-generation antidepressants in the treatment of major depressive disorder[J].Annals of Internal Medicine,2005,143(6):415-426.
[41] DONG X,YANG C,CAO S,et al.Tea consumption and the risk of depression:A meta-analysis of observational studies[J].Australian and New Zealand Journal of Psychiatry,2015,49(4):334-345.
[42] YIN C,GOU L,LIU Y,et al.Antidepressant‐like effects of L-theanine in the forced swim and tail suspension tests in mice[J].Phytotherapy Research,2011,25(11):1636-1639.
[43] 杨怡.茶氨酸对抑郁模型大鼠行为学、神经递质的影响[D].长沙:中南大学,2013.YANG Y.The effects of L-theanine on behavior and neurotransmitter of depression model rats [D].Changsha:Central South University,2013.
[44] SHEN M,YANG Y,WU Y,et al.L‐theanine ameliorate depressive-like behavior in a chronic unpredictable mild stress rat model via modulating the monoamine levels in limbic-cortical-striatal-pallidalthalamic-circuit related brain regions[J].Phytotherapy Research,2019,33(2):412-421.
[45] WANG W,HU X,ZHAO Z,et al.Antidepressant-like effects of liquiritin and isoliquiritin from Glycyrrhiza uralensis in the forced swimming test and tail suspension test in mice[J].Progress in Neuro-Psychopharmacology and Biological Psychiatry,2008,32(5):1184.
[46] DHINGRA D,SHARMA A.Antidepressant-like activity of Glycyrrhiza glabra L.in mouse models of immobility tests[J].Progress in Neuropsychopharmacology &Biological Psychiatry,2006,30(3):449-454.
[47] YI L T,LI J M,LI Y C,et al.Antidepressantlike behavioral and neurochemical effects of the citrus-associated chemical apigenin[J].Life Sciences,2008,82(13/14):741-751.
[48] YING X,KU B S,YAO H Y,et al.The effects of curcumin on depressive-like behaviors in mice[J].European Journal of Pharmacology,2005,518(1):40-46.
[49] APOSTOLOPOULOS V,STOJANOVSKA L,MIKKELSEN K.The effects of vitamin B in depression[J].Current Medicinal Chemistry,2016,23(38):4317-4337.
[50] WU Y,ZHANG L,LI S,et al.Associations of dietary vitamin B1,vitamin B2,vitamin B6,and vitamin B12 with the risk of depression:A systematic review and meta-analysis[J].Nutrition Reviews,2022,80(3):351-366.
[51] HVAS A M,JUUL S,BECH P,et al.Vitamin B6 level is associated with symptoms of depression[J].Psychother Psychosom,2004,73(6):340-343
[52] ODAI T,TERAUCHI M,SUZUKI R,et al.Depressive symptoms in middle-aged and elderly women are associated with a low intake of vitamin B6:A cross -sectional study [J].Nutrients,2020,12(11):3437.
[53] 刘昊,徐爱军,秦丽娟,等.人参皂苷Rb1 对抑郁症大鼠海马5-HT 及5-HT1A 受体表达的影响[J].时珍国医国药,2014,25(11):2565-2567.LIU H,XU A J,QIN L J,et al.Influence of ginsenoside Rb1 on 5-HT level and expression of 5-HT1A receptor in hippocampus of depressive rats[J].Lishizhen Medicine and Materia Medica Research,2014,25(11):2565-2567.
[54] ZANELATI T V,BIOJONE C,MOREIRA F A,et al.Antidepressant -like effects of cannabidiol in mice:Possible involvement of 5-HT1A receptors[J].British Journal of Pharmacology and Chemotherapy,2010,159(1):122-128.
[55] ZHU G,YANG S,XIE Z,et al.Synaptic modification by L-theanine,a natural constituent in green tea,rescues the impairment of hippocampal longterm potentiation and memory in AD mice[J].Neuropharmacology,2018,138:331-340.
[56] BERG J M.Zinc fingers and other metal-binding domains.Elements for interactions between macromolecules[J].Journal of Biological Chemistry,1990,265(12):6513.
[57] SZEWCZYK B,POLESZAK E,WLA P,et al.The involvement of serotonergic system in the antidepressant effect of zinc in the forced swim test [J].Progress in Neuro-Psychopharmacology and Biological Psychiatry,2009,33(2):323-329.
[58] BARRONDO S,SALLÉS J.Allosteric modulation of 5-HT(1A)receptors by zinc:Binding studies[J].Neuropharmacology,2009,56(2):455-462.
[59] SATAŁA G,![]() B,LENDA T,et al.Allosteric inhibition of serotonin 5-HT7 receptors by zinc ions[J].Molecular Neurobiology,2018,55(4):2897-2910.
B,LENDA T,et al.Allosteric inhibition of serotonin 5-HT7 receptors by zinc ions[J].Molecular Neurobiology,2018,55(4):2897-2910.
[60] SATAA G,![]() B,STACHOWICZ K,et al.Concentration-dependent dual mode of Zn action at serotonin 5-HT1A receptors:In vitro and in vivo studies[J].Molecular Neurobiology,2015,53(10):6869-6881.
B,STACHOWICZ K,et al.Concentration-dependent dual mode of Zn action at serotonin 5-HT1A receptors:In vitro and in vivo studies[J].Molecular Neurobiology,2015,53(10):6869-6881.
[61] WANG D,GAO Q,ZHAO G,et al.Protective effect and mechanism of theanine on lipopolysaccharide-induced inflammation and acute liver injury in mice[J].Journal of Agricultural and Food Chemistry,2018,66(29):7674-7683.
[62] WANG D,CAI M,WANG T,et al.Theanine supplementation prevents liver injury and heat shock response by normalizing hypothalamic-pituitaryadrenal axis hyperactivity in mice subjected to whole body heat stress[J].Journal of Functional Foods,2018,49:181-189.
[63] INOUE K,MIYAZAKI Y,UNNO K,et al.Stable isotope dilution HILIC‐MS/MS method for accurate quantification of glutamic acid,glutamine,pyroglutamic acid,GABA and theanine in mouse brain tissues[J].Biomedical Chromatography,2016,30(1):55-61.
[64] MOU Z,HUANG Q,CHU S F,et al.Antidepressive effects of ginsenoside Rg1 via regulation of HPA and HPG axis [J].Biomedicine &Pharmacotherapy,2017,92:962.
[65] 蔡莉,李荣,吴清清,等.橙皮苷对慢性应激抑郁模型大鼠行为学及HPA 轴的影响[J].中国中药杂志,2013,38(2):229-233.CAI L,LI R,WU Q Q,et al.Influence of hesperidin on behavior and HPA axis in chronic stress and depression model rats[J].China Journal of Chinese Materia Media,2013,38(2):229-233.
[66] HU Y,LIAO H B,LIU P,et al.Antidepressant effects of piperine and its neuroprotective mechanism in rats[J].Journal of Chinese integrative medicine,2009,7(7):667-670.
[67] MESRIPOUR A,ALHIMMA F,HAJHASHEMI V,et al.The effect of vitamin B6 on dexamethasoneinduced depression in mice model of despair[J].Nutritional Neuroscience,2019,22(10):744-749.
[68] JURUENA M F,BOCHAROVA M,AGUSTINI B,et al.Atypical depression and non-atypical depression:Is HPA axis function a biomarker? A systematic review[J].Journal of Affective Disorders,2018,233:45-67.
[69] TAKEDA A,SAKAMOTO K,TAMANO H,et al.Facilitated neurogenesis in the developing hippocampus after intake of theanine,an amino acid in tea leaves,and object recognition memory[J].Cellular and Molecular Neurobiology,2011,31(7):1079-1088.
[70] LIAO D,LV C,CAO L,et al.Curcumin attenuates chronic unpredictable mild stress-induced depressive-like behaviors via restoring changes in oxidative stress and the activation of Nrf2 signaling pathway in rats[J].Oxidative Medicine and Cellular Longevity,2020,17:9268083.
[71] EL-FATTAH A A,FAHIM A T,SADIK N,et al.Resveratrol and dimethyl fumarate ameliorate depression-like behaviour in a rat model of chronic unpredictable mild stress[J].Brain Research,2018,1701:227-236.
[72] FREDERICKSON C J,KOH J Y,BUSH A I.The neurobiology of zinc in health and disease[J].Nature Reviews Neuroscience,2005,6(6):449-462.
[73] KAPLAN B J,CRAWFORD S G,FIELD C J,et al.Vitamins,minerals,and mood[J].Psychological Bulletin,2007,133(5):747-60.
[74] DIGIROLAMO A M,MANUEL R Z,WANG M,et al.Randomized trial of the effect of zinc supplementation on the mental health of school-age children in Guatemala[J].American Journal of Clinical Nutrition,2010(5):1241.
[75] SOLATI Z,JAZAYERI S,TEHRANI-DOOST M,et al.Zinc monotherapy increases serum brain-derived neurotrophic factor(BDNF)levels and decreases depressive symptoms in overweight or obese subjects:A double-blind,randomized,placebo-controlled trial[J].Nutritional Neuroscience,2015,18(4):162-168.
[76] NOWAK G,LEGUTKO B,SZEWCZYK B,et al.Zinc treatment induces cortical brain-derived neurotrophic factor gene expression[J].European Journal of Pharmacology,2004,492(1):57-59.
[77] FRANCO J L,POSSER T,BROCARDO P S,et al.Involvement of glutathione,ERK1/2 phosphorylation and BDNF expression in the antidepressant-like effect of zinc in rats[J].Behavioural Brain Research,2008,188(2):316-323.
[78] ![]() M,LEGUTKO B,SZEWCZYK B,et al.Antidepressant-like activity of zinc:Further behavioral and molecular evidence [J].Journal of Neural Transmission,2008,115(12):1621.
M,LEGUTKO B,SZEWCZYK B,et al.Antidepressant-like activity of zinc:Further behavioral and molecular evidence [J].Journal of Neural Transmission,2008,115(12):1621.
[79] KATARZYNA M,B BOGUSŁAWA,BIRGITTE H,et al.GPR39(zinc receptor)knockout mice exhibit depression-like behavior and CREB/BDNF downregulation in the hippocampus[J].International Journal of Neuropsychopharmacology,2015(3):337-338.
[80] MYNIEC K,DOBOSZEWSKA U,SZEWCZYK B,et al.The involvement of the GPR39-Zn2+-sensing receptor in the pathophysiology of depression.Studies in rodent models and suicide victims[J].Neuropharmacology,2014,79:290-297.
[81] 程瑞凤,华冰,景晶,等.甘草总黄酮抗大鼠应激抑郁行为作用及对海马脑区神经细胞凋亡调控相关蛋白表达的影响[J].中药药理与临床,2014(2):69-72.CHENG R F,HUA B,JING J,et al.Modulation of the apoptotic protein expression in hippocampus is associated with the antidepressant effects of licorice flavonoids from Glycyrrhiza uralensis in rats[J].Pharmacology and Clinics of Chinese Materia Medica,2014(2):69-72.
[82] CHEN M,ZHANG Q P,ZHU J X,et al.Involvement of FGF-2 modulation in the antidepressant-like effects of liquiritin in mice[J].European Journal of Pharmacology,2020,881:173297.
[83] FAN C,SONG Q,WANG P,et al.Neuroprotective Effects of Ginsenoside-Rg1 against depression-like behaviors via suppressing glial activation,synaptic deficits,and neuronal apoptosis in rats[J].Frontiers in Immunology,2018,9:2889.
[84] LI Y,WANG L,WANG P,et al.Ginsenoside-Rg1 rescues stress-induced depression-like behaviors via suppression of oxidative stress and neural inflammation in rats [J].Oxidative Medicine and Cellular Longevity,2020,2020:2325391.
[85] FAN C,SONG Q,WANG P,et al.Neuroprotective effects of curcumin on IL -1β -induced neuronal apoptosis and depression-like behaviors caused by chronic stress in rats[J].Frontiers in Celluar Neuroscience,2019,12:516.
[86] FAN C,LI Y,LAN T,et al.Prophylactic treatment of curcumin in a rat model of depression by attenuating hippocampal synaptic loss[J].Food Funct,2021,12(22):11202-11213.
[87] WANG Y S,SHEN C Y,JIANG J G.Antidepressant active ingredients from herbs and nutraceuticals used in TCM:Pharmacological mechanisms and prospects for drug discovery[J].Pharmacological Research,2019,150:104520.
[88] KANG A,XIE T,ZHU D,et al.Suppressive effect of ginsenoside Rg3 against lipopolysaccharideinduced depression-like behavior and neuroinflammation in mice[J].Journal of Agricultural and Food Chemistry,2017,65(32):6861-6869.
[89] ZHANG X,WANG G,GURLEY E C,et al.Flavonoid apigenin inhibits lipopolysaccharide -induced inflammatory response through multiple mechanisms in macrophages[J].PloS One,2014,9(9):e107072.
[90] LI R,WANG X,QIN T,et al.Apigenin ameliorates chronic mild stress-induced depressive behavior by inhibiting interleukin-1β production and NLRP3 inflammasome activation in the rat brain [J].Behavioural Brain Research,2016,296:318-325.
[91] REZAI-ZADEH K,EHRHART J,YUN B,et al.Apigenin and luteolin modulate microglial activation via inhibition of STAT1-induced CD40 expression[J].Journal of Neuroinflammation,2008,5(1):41.
[92] ZHANG W Y,GUO Y J,HAN W X,et al.Curcumin relieves depressive-like behaviors via inhibition of the NLRP3 inflammasome and kynurenine pathway in rats suffering from chronic unpredictable mild stress [J].International Immunopharmacology,2019,67:138-144.
[93] REYNOLDS E.Vitamin B12,folic acid,and the nervous system[J].Lancet Neurology,2006,5(11):949-960.
[94] ROBERTA,FUSCO,ROSALBA,et al.Melatonin plus folic acid treatment ameliorates reserpine-induced fibromyalgia:An evaluation of pain,oxidative stress,and inflammation [J].Antioxidants(Basel,Switzerland),2019,8(12):628
[95] KUPFER D J,FRANK E,PHILLIPS M L.Major depressive disorder:New clinical,neurobiological,and treatment perspectives[J].Lancet.2012,379(9820):1045-1055.
[96] OTTE C,GOLD S M,PENNINX B W,et al.Major depressive disorder[J].Nature Reviews Disease Primers,2016,15(2):16065.