益生菌是一类活的微生物,当摄入一定量时,能给宿主的健康带来益处[1]。其种类多样,主要分为乳杆菌属和双歧杆菌属[2-3]。研究表明,益生菌有改变肠道微生物群组成,产生短链脂肪酸和共轭亚油酸,降解毒素及其各自的受体,促进消化并改善微量营养素吸收的能力[4],从而对肥胖[5]、肠易激综合征[6]、免疫调节[7]、癌症[8]和溃疡性结肠炎[9-10]有一定的预防作用。然而,益生菌在使用过程中必须保持活性,以提供更多的健康益处。
由于益生菌有诸多益处,因此目前被广泛应用到食品中。以奶制品和膳食补充剂为主要销售形式[11],奶制品以液态奶和酸奶为主,膳食补充剂的常见产品类型有胶囊、粉剂、片剂、口服液等。近几年来,益生菌产品的市场虽呈现逐步上升的趋势,但益生菌的活性也是不容忽视的问题[12]。目前益生菌应用中亟需解决的问题是:提高益生菌在胃液和肠液等极端环境的存活率,保证益生菌在肠道中达到一定数量(107 CFU/g);实现益生菌控制释放与靶向运输,提高其生物有效性。
研究学者尝试不同的方法来增强和保持益生菌细胞的活力。如通过大量试验筛选具有消化道抗性的益生菌菌株或者通过构建基因菌株来增加益生菌对消化道极端环境的抵抗性,使用耐氧容器避免益生菌与氧气接触,加入能够刺激益生菌活力的微量营养素(例如低聚果糖),超声波处理细菌和微囊化[13]。其中,益生菌微胶囊的应用最为广泛,是目前国内外研究的热点,且被认为是最为高效和经济的保护益生菌活性的方法之一。
1 益生菌微胶囊
益生菌微胶囊是指通过一定的技术手段将益生菌包埋在密闭的材料中,形成微小粒子。微胶囊壁所用的材料被称作壁材,被包裹在内部的益生菌通常被叫做芯材,芯材可以是单一的益生菌、复合的益生菌,也可以是益生菌与一些功能性成分,如益生元、营养物质等[14]。益生菌微胶囊的包埋效果因壁材的类型和包埋方式而异。
1.1 益生菌微胶囊壁材
壁材的选用是微胶囊包埋技术的关键,表1总结了近5 年Web of Science 和中国知网收录的关于包埋益生菌的壁材及文章篇数。
表1 包埋益生菌的壁材及文章数量
Table 1 Wall materials and number of articles of embedded probiotics
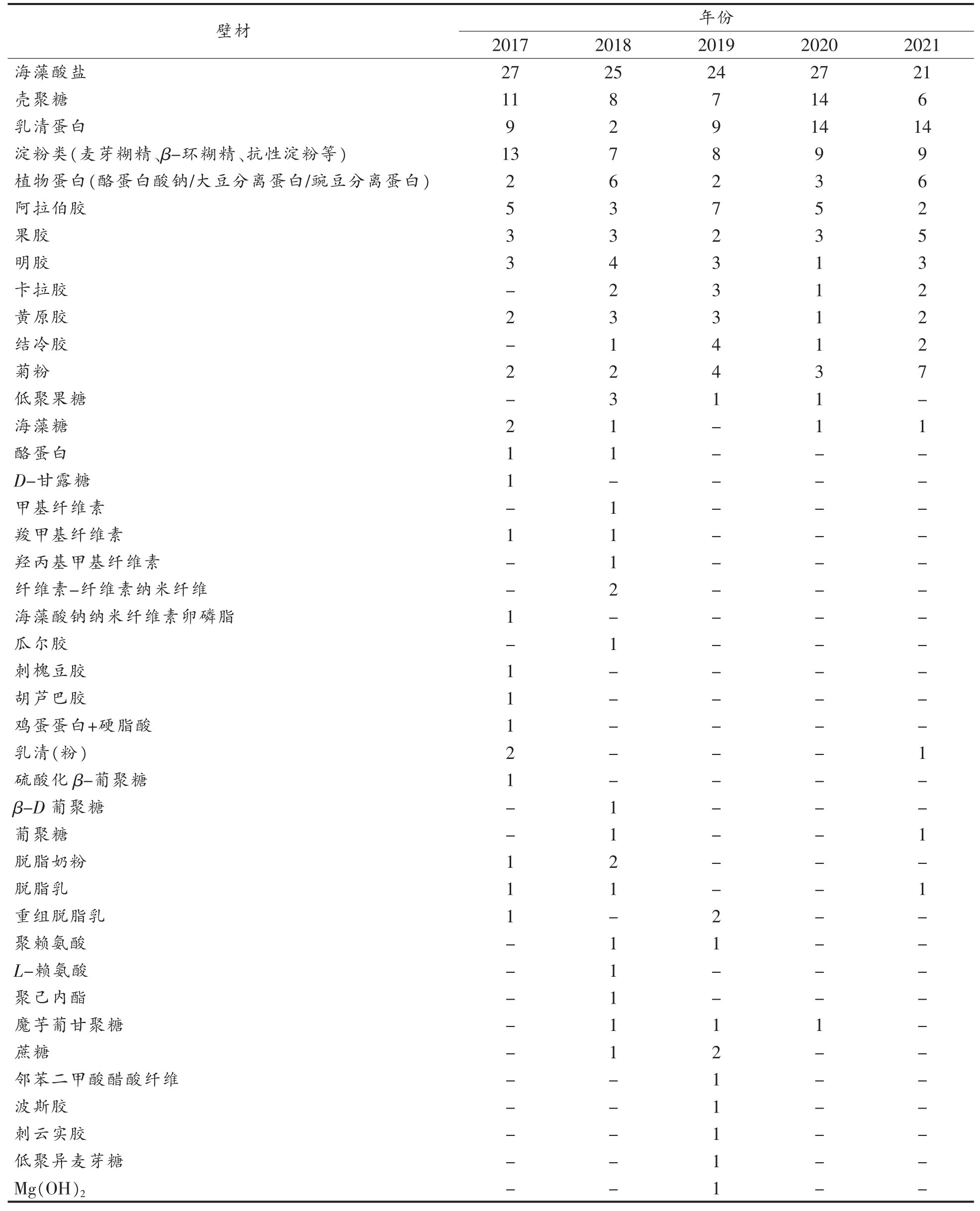
(续表1)
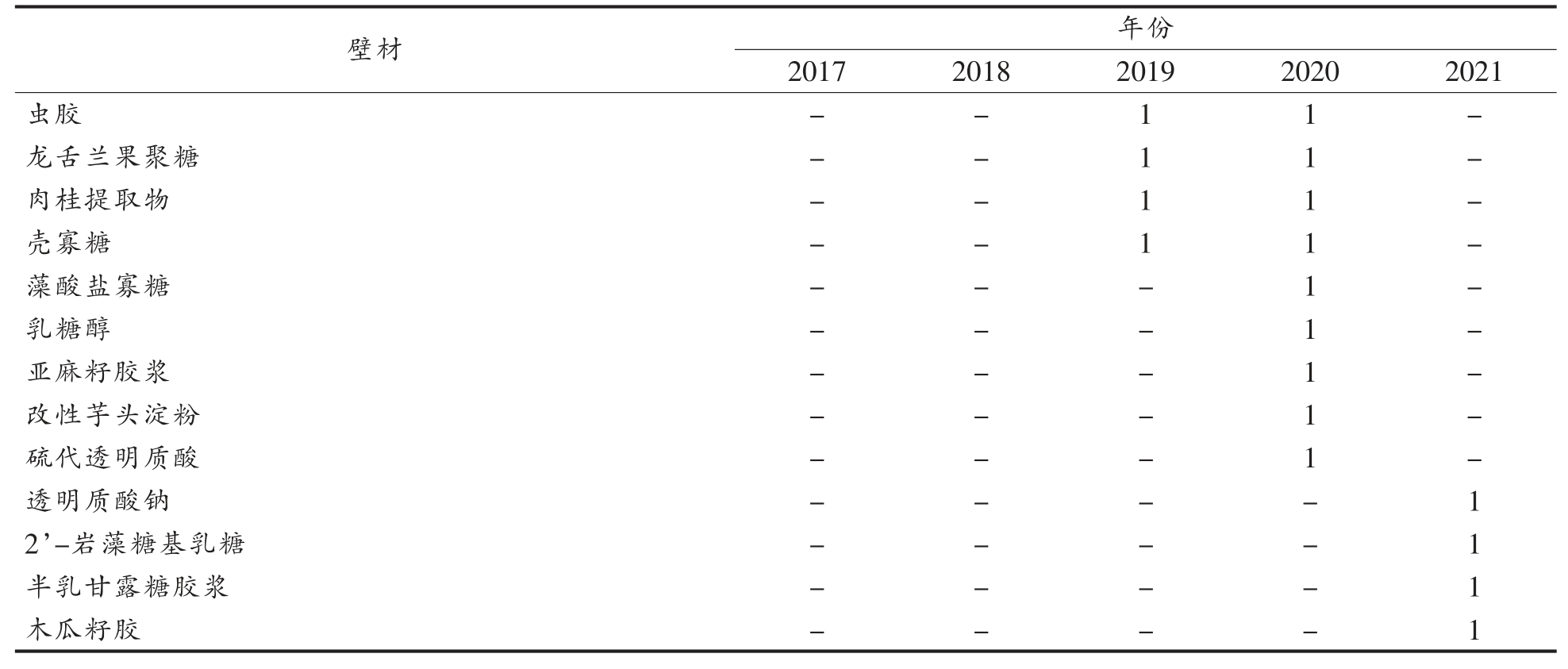
由表1 可知,越来越多的材料被用作壁材。最常见的包埋材料是海藻酸盐、壳聚糖、乳清蛋白、淀粉类、植物蛋白、阿拉伯胶、果胶、明胶、卡拉胶和黄原胶。还有一些益生元成分也被用作壁材,菊粉、低聚果糖和抗性淀粉是较常见的3 种益生元,它们可以与益生菌协同,进而作为某些益生菌的增殖因子,维持优势菌的地位。此外,还开发一些新型壁材,如魔芋葡甘聚糖、硫代透明质酸、岩藻糖、透明质酸钠等。
一种材料可用于构建微胶囊,将2 种甚至多种材料结合使用对增强其性能更为有利。海藻酸钙和乳清分离蛋白混合制备的益生菌微胶囊可在模拟消化液中更有效地保护益生菌[15];海藻酸盐和壳聚糖这类离子多糖常用作乳杆菌、双歧杆菌的微胶囊壁材,这两种壁材所形成的多层膜结构或者微球形结构是一种高度稳定的聚电解质复合物体系,对胃肠道消化条件有明显改善作用,可实现肠道中完全释放[16-17];还可以提高益生菌的稳定性,增强其黏膜黏附性能[18]。麦芽糊精单独使用不是一种很好的乳化剂,而其与蛋白质结合使用作为壁材,具有优异的乳化性能,可呈现出更好的凝胶分布和成膜性能[19-20]。此外,使用益生元作为涂层介质是最近的趋势,尤其在基于藻酸盐的胶囊中加入益生元将有助于益生菌的存活及其递送到人体内的作用靶点[21]。
由于微胶囊的包埋效果与其所使用的壁材紧密相关,因此,应充分了解每种壁材的性能(表2)。
表2 常见的几种壁材的特性
Table 2 Characteristics of several common wall materials

注:“-”表示未提到。
1.2 不同类型壁材对益生菌活性及肠道释放能力的影响
微胶囊化是食品工业中保护益生菌免受胃酸和肠道胆汁盐条件影响的有效方法,然而,选用不同壁材制备的微胶囊对益生菌的活性、胃肠道耐受性和肠道输送的影响均不同。Khorshidi 等[30]利用乳清蛋白和黄原胶涂层在嗜酸乳杆菌微囊表面,提高了益生菌在模拟胃肠道条件下的活力。刘月静等[31]利用海藻酸钠和刺云实胶制备的鼠李糖乳杆菌微胶囊能抵抗胃酸环境,实现肠靶向性释放。Calinescu 等[32]制备的羧甲基高直链淀粉和壳聚糖的共混膜包衣片,可为鼠李糖乳杆菌提供有效保护。其中羧甲基高直链淀粉提高了活菌在胃部的稳定性,壳聚糖实现了活菌的延迟释放及结肠靶向输送。类似地,Zaeim 等[33]将植物乳杆菌和乳双歧杆菌分别与抗性淀粉和菊糖共包埋到单一海藻酸盐/壳聚糖基质中,其中,含有菊糖的微胶囊在储存期间表现出更好的活力,而含抗性淀粉的微胶囊在胃肠道条件下对益生菌的存活率更高,这说明高分子质量益生元能促进益生菌在胃肠道条件下的靶向递送和存活。
1.3 益生菌微胶囊的包埋技术
包埋技术也会影响益生菌微胶囊的包埋效果。迄今为止,已经探索了多种技术来包埋益生菌。主要通过挤压、乳化、凝聚、喷雾干燥、冷冻干燥以及流化床来完成[34]。如表3 所示,每种技术都有其优缺点。在挤压法中,将含有益生菌的亲水胶体溶液倒入硬化溶液中,并使用高温干燥细菌细胞,这会影响细菌细胞的存活率。乳化方法需要使用植物油,这会导致胶囊大小和形状的变化,且细胞是湿收集的。喷雾干燥比冷冻干燥便宜,然而由于脱水和热的存在,细胞的活力损失非常高。相对而言,冷冻干燥对细胞的热损伤最小,然而,必须使用冷冻保护剂来抑制对细胞的冷损伤。此外,这些包埋技术的其它缺点是封装效率和负载能力较低,粒径分布较宽。在选择益生菌微胶囊的包埋方式时,要综合考虑所要包埋芯材的特性以及所选壁材的性质,从而能够最大限度的提高益生菌的包埋率和细胞活力,以有助于发挥益生作用[14]。
表3 益生菌不同包埋技术的优缺点
Table 3 Advantages and disadvantages of the different embedding technologies of probiotics
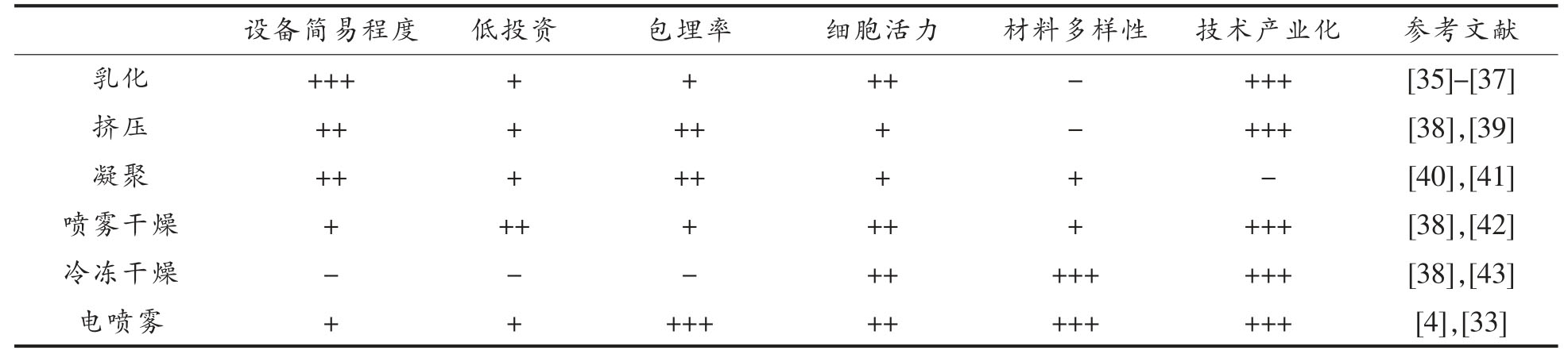
注:“+++”表示最大值;“-”表示不存在。
近年来,电喷雾已经成为益生菌的替代包埋技术,其主要原理是聚合物溶液在通过针头时,由于针尖上施加的高电势电场而带电,使其被雾化成细的液滴,然后这些带电的液滴落向带相反电荷的金属收集器,利用该收集器形成微粒(图1)。电喷雾法在不需要有害溶剂的情况下,可使用范围广泛的壁材料制备高存活率的微胶囊。Phuong等[44]使用电喷雾技术对载有植物乳杆菌的微胶囊进行了胃肠递送评价,发现壳聚糖包覆的海藻酸盐颗粒在胃黏膜上表现出优异的保留能力。
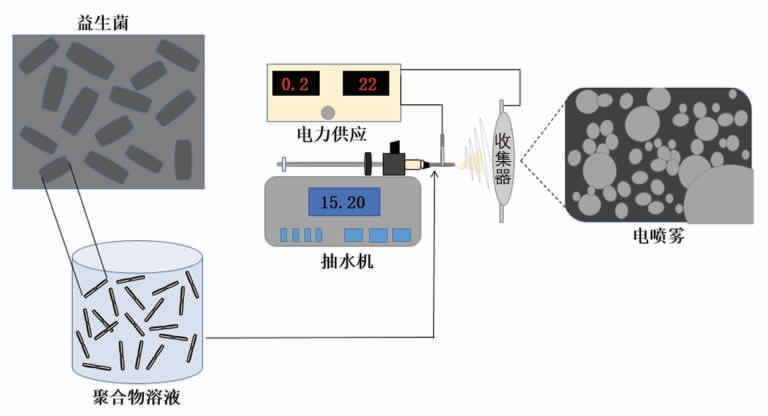
图1 益生菌电喷雾包埋示意图
Fig.1 Schematic diagram of electrospray embedding for probiotics
1.4 不同包埋技术对益生菌活性及肠道释放能力的影响
研究表明,在选用同种壁材的情况下,不同包埋方式制备的微胶囊,在益生菌活性和益生菌肠道释放的情况不同。Pupa 等[45]比较了挤压、乳化和喷雾干燥方法制备的海藻酸盐和壳聚糖双涂层微胶囊的特性,发现在室温下储存6 个月后,喷雾干燥颗粒的益生菌活力在3 种方法中最高。周莉等[46]以海藻酸钠和乳清蛋白为壁材,采用挤压法制备的微胶囊经人工胃液处理后菌体存活率≥70%,在模拟肠液中60 min 后能够完全释放出来;而Dehkordi 等[47]以相同壁材利用乳化-内凝胶技术制备的微胶囊,在模拟胃肠消化120 min 的条件下,胃中存活率为87%,120 min 后益生菌释放数量为8.22 lg(CFU/mL),实现了在肠靶向释放。
综上所述,微胶囊化为益生菌提供了物理屏障,有助于抵御或者延缓胃酸、胆盐等有害物质的渗透,使其免受食品加工、储存和食用后在胃肠道内消化等不利环境的影响,提高益生菌存活率,保证益生菌的活性。然而,不同类型壁材和包埋技术对益生菌的活性和肠道输送性能不同。因此,制备微胶囊时,应在个案基础上分析选择壁材和包埋技术的组合,从而发挥益生菌的最大效用。
2 益生菌微胶囊靶向递送
益生菌的递送可以调节肠道菌群,从而可能影响某些人类疾病的治疗。因此,通过口服益生菌调节肠道菌群时,益生菌微胶囊必须缓慢释放,以确保能将其顺利输送至消化道远端。然而,以往的胶囊化研究大多是为了维持益生菌在消化液中的存活率,只有少数研究涉及到益生菌微胶囊的具体释放区域。如Vos 等[48]指出,为了刺激和调节免疫应答,应当在肠道产生免疫信号的区域释放益生菌。Jiménez-Pranteda 等[49]利用益生菌胞外多糖作为植物乳杆菌微胶囊的涂层,实现植物乳杆菌的结肠靶向性释放。近年来,越来越多的研究表明利用微胶囊或涂膜包衣可以将益生菌输送至结肠(图2),起到把活菌载运并定植于结肠的作用。
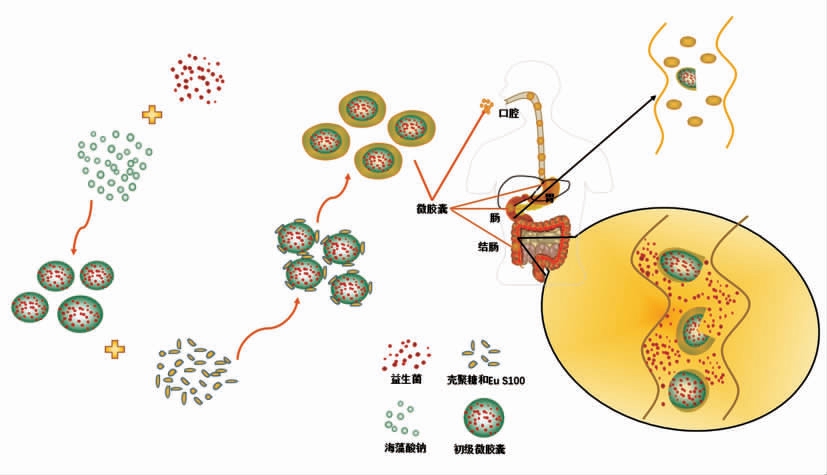
图2 微胶囊化益生菌的靶向递送过程
Fig.2 Targeted delivery process of microencapsulated probiotics
2.1 靶向递送的类型
根据结肠的生理学特点,研究人员设计出了不同的靶向递送体系。目前,依据递送原理的不同,将靶向递送体系主要分为以下几种类型:
2.1.1 pH 值敏感型递送体系 该体系的原理主要是基于胃肠道pH 值的差异,以pH 值敏感型材料为外壳,搭载益生菌于其内部,在低pH 值的胃肠道上段和pH 值为6.8 的小肠中,外壳材料不溶解,内容物释放较少,然而到达高pH 值(7.4)的结肠部位时,外壳材料逐渐溶解,益生菌释放量增加,从而实现靶向递送的目的。研究表明,pH 值敏感型被广泛用于肠道药物输送。Li 等[50]开发了一种新型肠道靶向载体,用于胃中乳酸菌的pH 值响应保护和小肠中乳酸菌快速释放,由海藻酸钙和鱼精蛋白复合壁材包裹的干酪乳杆菌(Lactobacillus casei CICC 23185)微胶囊。在肠液中,由于胰蛋白酶与鱼精蛋白的作用导致其在50 min内全部释放,而普通海藻酸钙包裹的微胶囊释放所耗时是它的7.6 倍。类似地,Qin 等[51]将植物乳杆菌菌株液体包裹在具有pH 值敏感海藻酸钙-EDTA 体系的W1/O/W2 双乳剂中,在模拟GIT(胃、小肠和结肠)消化后,益生菌在结肠中的活性基本没有损失,实现了结肠靶向递送,说明了具有pH 值敏感海藻酸钙-EDTA 体系的W1/O/W2 双乳剂可作为益生菌的结肠靶向释放载体。
2.1.2 菌群/酶触型递送体系 菌群/酶触型递送体系的原理是结肠环境存在大量的微生物群,这些微生物群可代谢出不同的酶,例如β-葡萄糖糖苷酶、纤维素酶、偶氮还原酶等。许多如多糖类的高分子材料壳聚糖、葡聚糖、海藻酸钠、硫酸软骨素、果胶等在胃和小肠中不会被降解,而在结肠中可被微生物群代谢产生的特定的酶降解,可利用此类高分子材料制备成不同形式的载体,从而实现益生菌的结肠靶向递送。
Cheng 等[52]以海藻酸钠和鱼精蛋白为原料,通过静电液滴与层层自组装相结合,制备了酶触发熔丝状微胶囊,在口服给药过程中,含两层鱼精蛋白的海藻酸盐微胶囊保护益生菌免受酸性和胆盐的伤害,控制细胞释放,并在胰蛋白酶的催化下层层分解成短的精氨酸基肽,因鱼精蛋白降解的肽对肠上皮细胞具有高效易位活性,这进一步提高了细菌的黏附强度,从而使益生菌成功地在结肠定植。
2.1.3 黏附型递送体系 该体系的原理是基于结肠黏膜具有独特且强大的结合能力,利用巯基化聚合物包裹益生菌,因硫化聚合物与黏液糖蛋白表面半胱氨酸形成稳定的二硫键,增加其在肠道的黏附性,从而实现益生菌的结肠靶向递送。此类递送体系不仅增强了胃酸抵抗力,而且还增加了肠道黏附定植能力。Liu 等[53]制备了一种具有黏液黏附性的硫代氧化魔芋葡甘聚糖(sOKGM)微球,体内外试验证实,sOKGM 微球可以保护益生菌免受胃酸的影响,在肠道内响应性释放益生菌,并提供黏液黏附和定植特性。类似地,在Chen 等[54]的研究中,用硫代壳聚糖作为涂层的微胶囊比壳聚糖涂层的在结肠中滞留时间更长。
2.1.4 其它递送体系 除此之外,还有时滞型靶向递送体系和压力依赖型递送体系。时滞型靶向递送体系的原理主要是摄入体内的食物依次经过胃、小肠到达结肠一般需要5~6 h,虽然胃排空的时间是不规律的,会影响食物通过肠道的时间,但食物在小肠的通过时间相对规律,通常为3~4 h。在这种情况下,可以构建具有时间依赖性的结肠靶向递送体系,从而达到益生菌的缓释;压力依赖型递送体系的原理是由于结肠作为水分和电解质重吸收的重要部位,水分的重吸收会造成结肠内容物的稠度增大,当肠道蠕动时,肠壁对内容物会产生较大的压力,这种现象会迫使体系崩解释放益生菌。
2.2 靶向递送益生菌微胶囊的应用
国内外的研究已经证实了益生菌微胶囊的有效性、安全性和耐受性。利用微胶囊将益生菌输送到结肠中释放和定植,有助于预防高脂血症[55],治疗牙周炎[56]和代谢综合症[57],发挥抗氧化[58-59]和增强免疫功能[59]。此外,益生菌结肠靶向性微胶囊还能防治溃疡性结肠炎(UC),然而目前关于益生菌结肠靶向微胶囊对UC 防治的研究仍处于实验室阶段。房正邹[60]利用壳聚糖包衣双歧杆菌微胶囊使双歧杆菌实现肠靶向释放,可有效缓解结肠炎症状。Vishwakarma 等[61]将美沙拉嗪和益生菌包裹在果胶微球中,将其成功共递送到结肠并缓慢释放发挥抗结肠炎作用。
3 展望
综上所述,微胶囊壁材的发展越来越多元化,微囊化可有效提高益生菌活性,不仅能维持益生菌在贮藏过程中的稳定性;还能保护其通过消化道抵达结肠,提高活菌在肠道表面黏膜的定植能力。然而从目前的研究来看,仍然迫切需要进行临床研究,以验证微胶囊的长期安全性和有效性。除此之外,还应探究选用不同壁材和方法制备微胶囊的粒径能否控制在理想的范围内,粒径对包埋率、胃肠道存活率和靶向释放是否有影响。随着技术的发展,未来微胶囊技术能用于所有食品以及药品的载体输送,实现食物活性成分在结肠的控制释放并提高生物利用率,为结肠功能性食品的开发奠定基础。
[1] SABINA F,HEALTH P.Microorganisms with claimed probiotic properties:An overview of recent literature[J].International Journal of Environmental Research and Public Health,2014,11(5):4745-4767.
[2] YUSUKE T,TOKUNORI I,SHINYA S,et al.Association of probiotic clostridium butyricum therapy with survival and response to immune checkpoint blockade in patients with lung cancer[J].Cancer Immunology Research,2020,8(10):1236-1242.
[3] LIU M,XIE W J,WAN X Y,et al.Clostridium butyricum modulates gut microbiota and reduces colitis associated colon cancer in mice[J].International Immunopharmacology,2020,88:106862.
[4] MENDES A C,CHRONAKI I S.Electrohydrodynamic encapsulation of probiotics:A review[J].Food Hydrocolloids,2021,117:106688.
[5] RAHAYU E S,MARIYATUN M,MANURUNG N E P,et al.Effect of probiotic Lactobacillus plantarum Dad-13 powder consumption on the gut microbiota and intestinal health of overweight adults[J].World Journal of Gastroenterology,2021,27(1):107-128.
[6] SADRIN S,SENNOUNE S,GOUT B,et al.A 2-strain mixture of Lactobacillus acidophilus in the treatment of irritable bowel syndrome:A placebocontrolled randomized clinical trial[J].Digestive and Liver Disease,2020,52(5):534-540.
[7] SUN L L,TIAN W L,GUO X J,et al.Lactobacillus gasseri JM1 with potential probiotic characteristics alleviates inflammatory response by activating the PI3K/Akt signaling pathway in vitro[J].Journal of Dairy Science,2020,103(9):7851-7864.
[8] YUE Y C,YE K,LU J,et al.Probiotic strain Lactobacillus plantarum YYC-3 prevents colon cancer in mice by regulating the tumour microenvironment[J].Biomedicine &Amp;Pharmacotherapy,2020,127:102-116.
[9] XIA Y J,CHEN Y,WANG G Q,et al.Lactobacillus plantarum AR113 alleviates DSS -induced colitis by regulating the TLR4/MyD88/NF-κB pathway and gut microbiota composition[J].Journal of Functional Foods,2020,67:103854.
[10] LUO R,ZHANG J,ZHANG X Y,et al.Bacillus subtilis HH2 ameliorates TNBS-induced colitis by modulating gut microbiota composition and improving intestinal barrier function in rabbit model[J].Journal of Functional Foods,2020,74:104167.
[11] 曲巍.国内外益生菌发展现状[J].科学中国人,2017(11):180.QU W.Development status of probiotics at home and abroad[J].Scientific Chinese,2017(11):180.
[12] 倪伟超.探讨国内外益生菌产品的发展情况[J].现代食品,2020(9):38-39.NI W C.To explore the development of probiotic products at home and abroad[J].Modern Food,2020(9):38-39.
[13] SARKAR S.Approaches for enhancing the viability of probiotics:A review[J].British Food Journal,2010,112(4):329-349.
[14] 罗丹阳.益生菌的肠道输送技术研究[D].广州:华南理工大学,2020.LUO D Y.Study on intestinal delivery technology of probiotics[D].Guangzhou:South China University of Technology,2020.
[15] HAN C L,XIAO Y J,LIU E C,et al.Preparation of Ca-alginate-whey protein isolate microcapsules for protection and delivery of L.bulgaricus and L.paracasei[J].International Journal of Biological Macromolecules,2020,163:1361-1368.
[16] TATIANA B I,RICARDO V C,EVA S A,et al.Functional properties of Lactobacillus casei C24 improved by microencapsulation using multilayer double emulsion [J].Food Research International,2021,141:110136.
[17] LI W,LUO X G,SONG R,et al.Porous cellulose microgel particle:A fascinating host for the encapsulation,protection,and delivery of Lactobacillus plantarum [J].Journal of Agricultural and Food Chemistry,2016,64(17):3430.
[18] ANSELMO A C,MCHUGR K J,WEBSTER J,et al.Layer-by-layer encapsulation of probiotics for delivery to the microbiome[J].Advanced Materials,2016,28(43):9442-9442.
[19] ZHANG J,ROSENBERG Y,ROSENBERG M.Microencapsulation properties of wall systems consisting of WHPI and carbohydrates[J].AIMS Agriculture and Food,2018,3(1):66-84.
[20] VANISKI R,SILVA S C D,SILVA-BUZANELLO R A,et al.Improvement of Lactobacillus acidophilus La-5 microencapsulation viability by spraydrying with rice bran protein and maltodextrin[J].Journal of Food Processing and Preservation,2021,45(4):e15364.
[21] PHUONG T L,ERIKA B,OTILIA A,et al.Effects of various polysaccharides(alginate,carrageenan,gums,chitosan)and their combination with prebiotic saccharides(resistant starch,lactosucrose,lactulose)on the encapsulation of probiotic bacteria Lactobacillus casei 01 strain.[J].International Journal of Biological Macromolecules,2021,183:1136-1144.
[22] DAMODHARAN K,PALANIYANDI S A,YANG S H,et al.Co-encapsulation of lactic acid bacteria and prebiotic with alginate -fenugreek gum -locust bean gum matrix:Viability of encapsulated bacteria under simulated gastrointestinal condition and during storage time[J].Biotechnology and Bioprocess Engineering,2017,22(3):265-271.
[23] YAO M F,XIE J J,DU H J,et al.Progress in microencapsulation of probiotics:A review[J].Comprehensive Reviews in Food Science and Food Safety,2020,19(2):1-18.
[24] 元博.高存活率益生菌微胶囊的制备及特性研究[D].烟台:烟台大学,2019.YUAN B.Preparation and characterization of probiotic microcapsules with high survival rate[D].Yantai:Yantai University,2019.
[25] 李晴,唐文倩,谢柳佳,等.不同壁材包埋对益生菌性能的影响[J].食品与发酵科技,2020,56(6):92-99.LI Q,TANG W Q,XIE L J,et al.Effects of different wall materials on the performance of probiotics[J].Food and Fermentation Technology,2020,56(6):92-99
[26] 栾倩.益生菌纤维素基微胶囊的构建及初步评价[D].北京:中国农业科学院,2018.LUAN Q.Construction and preliminary evaluation of probiotic cellulose based microcapsules[D].Beijing:Chinese Academy of Agricultural Sciences,2018.
[27] ZHANG X Y,ZHANG B,GE X Z,et al.Fabrication and characterization of whey protein-citrate mung bean starch-capsaicin microcapsules by spray drying with improved stability and solubility[J].Foods,2022,11(7):1049.
[28] GUL O,ATALAR I.Different stress tolerance of spray and freeze dried Lactobacillus casei Shirota microcapsules with different encapsulating agents[J].Food Science and Biotechnology,2019,28(3):807-816.
[29] JUNG J Y,ARNOLD R D,WICKER L.Pectin and charge modified pectin hydrogel beads as a colon-targeted drug delivery carrier[J].Colloids &Surfaces B Biointerfaces,2013,104:116-121.
[30] KHORSHIDI M,HESHMATI A,TAHERI M,et al.Effect of whey protein-and xanthan-based coating on the viability of microencapsulated Lactobacillus acidophilus and physiochemical,textural,and sensorial properties of yogurt[J].Food Science &Nutrition,2021,9(7):3942-3953.
[31] 刘月静,闫杨,王帅珂,等.鼠李糖乳杆菌肠溶性微胶囊的制备研究[J].聊城大学学报(自然科学版),2019,32(4):34-40.LIU Y J,YAN Y,WANG S K,et al.Preparation of enteric soluble microcapsules of Lactobacillus rhamnosus[J].Journal of Liaocheng University(Natural Science Edition),2019,32(4):34-40
[32] CALINESCU C,MATEESCU M A.Carboxymethyl high amylose starch:Chitosan self-stabilized matrix for probiotic colon delivery[J].European Journal of Pharmaceutics and Biopharmaceutics,2008,70(2):582-589.
[33] ZAEIM D,SARABI-JAMAB M,GHORANI B,et al.Double layer co-encapsulation of probiotics and prebiotics by electro-hydrodynamic atomization[J].LWT-Food Science and Technology,2019,110:102-109.
[34] RODRIGUES F J,CEDRAN M F,BICAS J L,et al.Encapsulated probiotic cells:Relevant techniques,natural sources as encapsulating materials and food applications -A narrative review[J].Food Research International,2020,137:109682.
[35] CEJA-MEDINA L I,ORTIZ-BASURTO R I,MEDINA-TORRES L,et al.Microencapsulation of Lactobacillus plantarum by spray drying with mixtures of Aloe vera mucilage and agave fructans as wall materials[J].Journal of Food Process Engineering,2020,43(8):e13436.
[36] ALFARO-GALARZA O,LÓPEZ-VILLEGAS E O,RIVERO-PEREZ N,et al.Protective effects of the use of taro and rice starch as wall material on the viability of encapsulated Lactobacillus paracasei subsp.Paracasei[J].LWT,2019,117:108686.
[37] AGUDELO J,CANO A,GONZALEZ-MAINEZ C,et al.Disaccharide incorporation to improve survival during storage of spray dried Lactobacillus rhamnosus in whey protein-maltodextrin carriers[J].Journal of Functional Foods,2017,37:416-423.
[38] 陈婷,王玉华,蔡丹,等.益生菌微胶囊技术研究进展[J].中国乳品工业,2016,44(1):31-37.CHEN T,WANG Y H,CAI D,et al.Research progress of probiotic microcapsule technology[J].China Dairy Industry,2016,44(1):31-37
[39] LILIANA L,MIRCEA O.Influence of different prebiotics on viability of Lactobacillus casei,Lactobacillus plantarum and Lactobacillus rhamnosus en capsulated in alginate microcapsules[J].Foods,2021,10(4):710.
[40] SILVA T M D,BARIN J S,LOPES E J,et al.Development,characterization and viability study of probiotic microcapsules produced by complex coacervation followed by freeze-drying[J].Ciência Rural,2019,49(7):e20180775.
[41] SILVA T M D,LOPES E J,CODEVILLA C F,et al.Development and characterization of microcapsules containing Bifidobacterium Bb-12 produced by complex coacervation followed by freeze drying[J].LWT,2017,90:412-417.
[42] QI W T,LIANG X X,YUN T T,et al.Growth and survival of microencapsulated probiotics prepared by emulsion and internal gelation[J].Journal of Food Science and Technology,2019,56(3):1-7.
[43] VISHALI D A,MONISHA J,SIVAKAMASUNDARI S K,et al.Spray freeze drying:Emerging applications in drug delivery[J].Journal of Controlled Release,2019,300:93-101.
[44] PHUONG T L,BUJNA E,KUN S,et al.Electrosprayed mucoadhesive alginate -chitosan microcapsules for gastrointestinal delivery of probiotics[J].International Journal of Pharmaceutics,2021,597:120342.
[45] PUPA P,APIWATSIRI P,SIRICHOKCHATCHA -WAN W,et al.The efficacy of three double-microencapsulation methods for preservation of probiotic bacteria[J].Scientific Reports,2021,11(1):13757.
[46] 周莉,王晓瑞,谭静,等.海藻酸钠-乳清蛋白复合益生菌微胶囊的构建及性能评价[J].中国食品添加剂,2020,31(4):108-113.ZHOU L,WANG X R,TAN J,et al.Construction and performance evaluation of sodium alginate whey protein composite probiotic microcapsules[J].China Food Additives,2020,31(4):108-113
[47] DEHKORDI S S,ALEMZADEH I,VAZIRI A S,et al.Optimization of alginate-whey protein isolate microcapsules for survivability and release behavior of probiotic bacteria[J].Applied Biochemistry and Biotechnology,2020,190(1):1-15.
[48] VOS P D,FAAS M M,SPASOJEVIC M,et al.Encapsulation for preservation of functionality and targeted delivery of bioactive food components[J].International Dairy Journal,2010,20(4):292-302.
[49] JIMÉNEZ-PRANTEDA M L,AGUILERA M,MCCARTNEY A L.Investigation of the impact of feeding Lactobacillus plantarum CRL 1815 encapsulated in microbially derived polymers on the rat faecal microbiota [J].Journal of Applied Microbiology,2012,113(2):399-410.
[50] LI M,HE F,ZHOU R Q,et al.Novel intestinaltargeted Ca-alginate-based carrier for pH-responsive protection and release of lactic acid bacteria[J].ACS Appl Mater Inter,2014,6(8):5962-5970.
[51] QIN X S,LUO Z G,LI X L.An enhanced pHsensitive carrier based on alginate-Ca-EDTA in a set-type W1/O/W2 double emulsion model stabilized with WPI-EGCG covalent conjugates for probiotics colon-targeted release[J].Food Hydrocolloids,2021,113:106460.
[52] CHENG Q K,LIU L,XIE M Z,et al.A colontargeted oral probiotics delivery system using an enzyme-triggered fuse-like microcapsule[J].Advanced Healthcare Materials,2021,10(8):2001953.
[53] LIU Y,LIU B,LI D,et al.Improved gastric acid resistance and adhesive colonization of probiotics by mucoadhesive and intestinal targeted konjac glucomannan microspheres[J].Advanced Functional Materials,2020,30(35):1-11.
[54] CHEN S,CAO Y,FERGUSON L R,et al.Evaluation of mucoadhesive coatings of chitosan and thiolated chitosan for the colonic delivery of microencapsulated probiotic bacteria[J].Journal of Microencapsulation,2013,30(2):103-15.
[55] YAO C Q,TIAN W J,SONG J J,et al.The antihyperlipidemic effect of microencapsulated Lactobacillus plantarum LIP-1 on hyperlipidemic rats[J].Journal of the Science of Food and Agriculture,2020,100(5):2007-2017.
[56] MIRTIC J,RIJAVEC T,ZUPANCIC S,et al.Development of probiotic-loaded microcapsules for local delivery:Physical properties,cell release and growth[J].European Journal of Pharmaceutical Sciences,2018,121:178-187.
[57] IQBAL U H,WESTFALL S,PRAKASH S.Novel microencapsulated probiotic blend for use in metabolic syndrome:Design and in-vivo analysis[J].Artificial Cells,2018,46(sup 3):1-9.
[58] REPALLY A,DASARI A,VENKATESAN A.Antiinflammatory and antioxidant properties of probiotic bacterium Lactobacillus mucosae AN1 and Lactobacillus fermentum SNR1 in Wistar albino rats[J].Frontiers in Microbiology,2018,9:3063.
[59] WANG Y W,DONG Z L,SONG D,et al.Effects of microencapsulated probiotics and prebiotics on growth performance,antioxidative abilities,immune functions,and caecal microflora in broiler chickens[J].Food &Agricultural Immunology,2018,29(1):1-11.
[60] 房正邹.构建CT 显影与肠溶靶向双功能微胶囊用于口服益生菌递送的研究[D].镇江:江苏大学,2017.FANG Z Z.Construction of CT developing and enteric targeting dual functional microcapsules for oral probiotic delivery[D].Zhenjiang:Jiangsu University,2017.
[61] VISHWAKARMA N,GANESHPURKAR A,PANDEY V,et al.Mesalazine-probiotics beads for acetic acid experimental colitis:Formulation and characterization of a promising new therapeutic strategy for ulcerative colitis[J].Drug Delivery,2015,22(1):94-99.