豆类是世界上食用最广泛的食物之一,有着近1 万年的悠久历史[1]。作为营养丰富的可持续食物资源,豆类是缓解粮食短缺的重要候选者。迄今为止,在1 300 多种的豆科植物中,只有20 种常被人们食用。联合国粮食及农业组织(Food and Agriculture Organization of the United Nations)将杂豆定义为除大豆、花生及作为蔬菜收获的豆类等高脂肪豆科植物之外的干收豆科作物,也称为干豆[2]。杂豆富含蛋白质、维生素和矿物质等营养成分,有较高的食用价值。作为世界杂豆生产大国,我国杂豆种类繁多,主要种属有绿豆、豌豆、红豆、云扁豆、豇豆及蚕豆等[3]。随着我国人民生活水平的提高,对杂豆的需求将显著增加,这与杂豆含有丰富的活性成分密不可分,其所富含的酚类化合物具有多种功能活性,例如抗氧化[4]、抗炎[5]和抗糖尿病[6]等。
酚类化合物是由莽草酸、苯丙烷和乙酸代谢途径合成的一大类次生代谢物,其种类繁多且结构复杂,是杂豆重要的活性化合物之一。酚类化合物的组成与含量影响杂豆的种皮颜色、感官特征及营养特性。如图1 所示,杂豆酚类化合物的种类主要由酚酸类、黄酮类、原花青素和香豆素等组成,并以游离、酯化或不溶性结合态的形式存在,其中不溶性结合酚类较为丰富,主要分布于杂豆的子叶和种皮部分[7]。表1 总结了常见杂豆的主要酚类化合物,可以发现原儿茶酸、没食子酸、对羟基苯甲酸、香豆酸是各品种杂豆最常见的酚类化合物,并且杂豆酚类化合物糖苷种类存在差异。由于基因型、环境及加工方式的影响,杂豆酚类化合物生物合成出现差异,并呈现出种类多、含量各异和功能活性多样性的趋势。
表1 常见杂豆的主要酚类化合物
Table 1 Main phenolic compounds in pulses

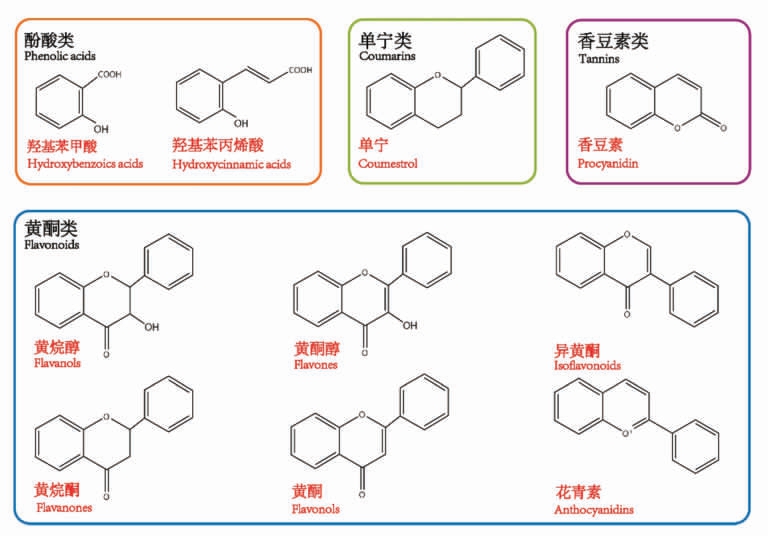
图1 杂豆主要酚类化合物分类和化学结构[8]
Fig.1 Classification and chemical structure of the main phenolic compounds of pulses[8]
本文概述影响杂豆酚类化合物多样性的因素,包括杂豆酚类化合物的提取、定性与定量方法。在此基础上,论述种属与产地、生长与贮藏过程及加工过程对杂豆酚类化合物多样性的影响,并阐述杂豆酚类化合物多样性面临的问题以及未来发展方向,旨在为杂豆酚类化合物多样性的形成机制及杂豆育种等研究提供理论参考。
1 酚类化合物的提取方法
由于酚类化合物提取的完整性直接影响其后续的定性、定量结果,因此酚类化合物的有效提取是多样性分析非常重要的环节。常用的有机溶剂萃取法能有效提取杂豆可溶性酚类化合物,而与细胞壁结合的酚类化合物,需要预先使用酸碱或酶水解法,将结合态酚类化合物释放出来。其次,有机溶剂的极性将决定溶剂系统对酚类化合物的选择性,进而影响杂豆总酚含量测定结果。黄豌豆、绿豆和鹰嘴豆的50%丙酮提取物总酚含量最高,而70%丙酮(+0.5%乙酸)溶液能有效提取黑豆、扁豆和红芸豆的酚类化合物[17]。另外,非极性溶剂更适用于提取豆类种子的酚类化合物[18],然而至今没有一种标准化的提取溶剂完全适用于植物材料全部酚类的提取[19]。
近年来,杂豆酚类化合物的提取方法正朝着清洁高效、影响更小的替代溶剂方向发展。新型提取方法主要通过缩短处理时间和降低能耗等方式,改善杂豆酚类化合物的提取效果,例如微波辅助萃取法[20]。总之,杂豆酚类化合物的提取效率取决于许多物理化学参数,提取参数的确定必须在酚类化合物的分配和避免结构破坏之间有合适的折中。然而,当酚类化合物以简单或高度聚合的结构形式存在,或者与其它各种植物基质成分形成复合物时,杂豆酚类化合物的提取难度将增大。需要依据样本类型及预期成分选择和调整提取方法,例如适当采用水解或消化步骤,以将共轭形式的酚类化合物释放为其苷元形式[21]。
2 酚类化合物的分析方法
杂豆酚类化合物的定性与定量是酚类化合物多样性研究中的重要环节,因为方法的选择直接影响结果的准确性,并涉及到多样性机理的探究。表2 总结了杂豆酚类化合物的定性与定量方法。一方面,二级管阵列检测器(DAD 或PDA)是最常用的两种液相检测器。另一方面,液相串联高分辨质谱技术常用于杂豆酚类化合物的鉴定,例如超高效液相串联飞行时间质谱(UPLC-QTOF-MS)。
表2 常见杂豆酚类化合物定性与定量方法比较
Table 2 Comparison of qualitative and quantitative methods for phenolic compounds of pulses

2.1 定性方法
色谱耦合各类液相检测器技术常用于杂豆酚类化合物的定性工作。液相检测器主要为紫外-可见光检测器(UV/vis)及二级管阵列检测器(DAD或PDA),而需要标准化合物对液相洗脱峰定性分析。此外,尽管单一的色谱峰面积定量分析能够直观展现酚类化合物的差异,但是容易忽略酚类化合物单体的变化趋势[31]。近年来,配备电喷雾离子源(ESI)和DAD 检测器的液相色谱串联高分辨质谱(HPLC-MS)对于鉴定杂豆酚类化合物至关重要,例如未知酚类化合物的表征[32]和糖苷键的识别[12]。另外,完善的液相色谱分离方法能够避免或减少酚类化合物异构体的共流出对定性结果的影响,例如反向C18 色谱柱可以有效分离蚕豆原花青素低聚物[35]。总体而言,基于光电检测器和质谱方法的鉴定结果只是临时的,需要与标准对照品相比较,然而大量购买酚类化合物标准品面临一定挑战。
2.2 定量方法
长期以来,Folin-Ciocalteu 比色法是量化杂豆总酚的主要方法,而其它干扰物质(VC、脱氧抗坏血酸及还原糖等)会与比色法所用的试剂反应,从而影响结果的准确性[36]。另外,UV/vis 可用于探究不同类别的酚类化合物含量差异,而无法解决最大吸收波长相同的酚类化合物共洗脱问题[37-38]。为解决上述问题,液相色谱-三重四极杆串联质谱的动态多反应监测扫描(MRM)模式可消除由共洗脱引起浓度过高的估计[39]。随着仪器与方法的发展,更多的方法开始应用于酚类化合物含量趋势的研究,例如基于基因分型芯片的全基因组关联分析可用于表征与杂豆总酚含量显著相关的遗传基因[40]。
3 影响杂豆酚类化合物多样性的因素
在杂豆的生长、贮藏和加工过程中,受多种外在因素影响,它的内外组织结构会发生不同形式的变化,直接影响酚类化合物的多样性。图2 可以看出,不同因素对杂豆酚类化合物含量的总体影响趋势存在差异。一方面,杂豆栽培涉及基因-环境的相互作用,许多重要的农艺性状和品质特性受到栽培条件的强烈影响,包括光照强度、气温、降水量和土壤特性等自然条件,以及灌溉方式、种植技术及人造光源等人为条件。另一方面,杂豆贮藏与加工过程伴随着酚类化合物的释放、降解和合成等过程。正是上述复杂多样的影响因素,使得杂豆酚类化合物含量与组成呈现出多样性变化。
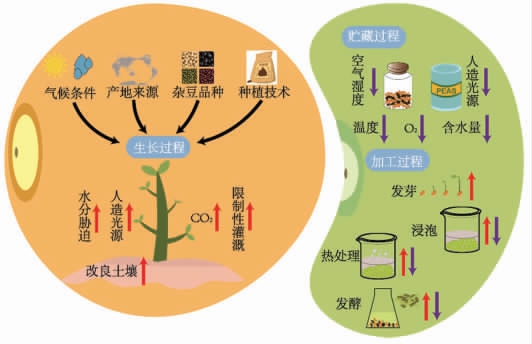
图2 不同因素影响杂豆酚类化合物含量的总体趋势
Fig.2 General trends in the phenolic content of pulses as influenced by different factors
3.1 种属与产地的影响
杂豆种属基因型的差异影响杂豆生长发育的转录表达及次生代谢产物合成与分解能力。如表3 所示,不同品种杂豆的总酚含量差异较大。种属差异影响着杂豆总酚含量和酚类化合物的组成,并且彩色种皮的杂豆有着较高的总酚含量,例如红色和斑驳种皮基因型菜豆的酚类化合物含量高于白色种皮基因型[41]。基因型决定杂豆的种皮颜色,并且每个基因座位上2 个等位基因的显性和隐性组合决定着4 种基本的种皮颜色,即棕色(Ggc Tgc)、灰色(Ggc tgc)、棕褐色(ggc Tgc)和绿色(ggc tgc)[42]。在以往的研究中已经证实基因型差异将赋予不同种皮颜色杂豆的酚类化合物组成差异,例如具有纯合隐形tgc 等位基因型的绿色和灰色扁豆的种皮,其黄酮-3-醇、原花青素和部分黄酮醇的相对含量较高[43]。来源于同一市场的同种颜色芸豆,其酚类化合物分布及组成仍然表现出相当大的差异,并且同一类别芸豆的遗传来源没有基本统一特征,较为合理的解释是杂豆发生基因趋同[44]。由于不同品种杂豆对环境条件的适应性差异,基因趋同可以使得受驯化的杂豆品种获得独特的性状与形貌特征,并表现出不同的优势性状,例如不受干旱条件影响的Gialet,以及总酚含量较高的Maron[45]。然而,现阶段的研究忽略了野生品种或栽培品种等地方品种的酚类化合物变化,更加关注的是改良品种,而栽培杂豆和野生杂豆的总酚含量受高遗传变异和环境的影响[46]。
表3 种属与产地对杂豆总酚含量的影响
Table 3 Effect of variety and origin on the total phenolic content of pulses

产地差异影响杂豆酚类化合物的含量与组成分布,主要的原因是不同气候条件及地理环境等自然因素导致与酚类生物合成相关的代谢通路差异表达,例如高水平的光照强度会增加脂质过氧化引起的损伤程度,酚类化合物的增多可能会为这种损害提供一定的保护,因为该机制与脯氨酸连接的戊糖磷酸途径对紫外线胁迫的反应有关[53-54]。栽培环境的差异导致不同品种杂豆的酚类化合物单体含量差异显著,例如槲皮素和金雀异黄素[55]。另外,遗传变异与环境的双重影响会对酚类化合物含量造成差异,例如有研究显示中国不同地区的75 个豌豆品种总酚含量范围为0.27~1.95 mg GAE/g dw[56]。
一般而言,不同品种杂豆生长在不同环境下的情况十分常见,此时基因型-环境的相互作用非常重要。环境因素对扁豆总酚含量的影响大于基因型效应,并且年份变化显著影响同一环境条件栽培杂豆的酚类化合物组成[57]。此外,高海拔生长环境迫使杂豆通过增加抗氧化物的合成来应对环境压力,使得高海拔栽培杂豆的酚类化合物含量更高[58]。同时,杂豆的高海拔种植往往带来高强度的紫外线照射,这会促使脂质过氧化损伤加重,进而激活酚类化合物生物合成通路[59],使得杂豆在生长过程中能合成更多的酚类化合物,例如高海拔种植的红小豆有着更高的酚类化合物含量[60]。
综上所述,品种和产地的影响更多地体现在与酚类化合物合成相关的代谢表达差异,进而影响杂豆最终的酚类化合物组成分布与含量。就杂豆品种与产地溯源而言,未来的研究应倾向于研究基因型、地理条件及气候等综合因素对酚类化合物富集的综合影响,不再仅仅局限于单一变量的研究工作。
3.2 生长与储藏过程的影响
众所周知,酚类化合物是植物次生代谢与防御反应的重要组成部分。非生物胁迫影响酚类化合物的生物合成过程,并引发胁迫感知等相关信号通路的激活[61-62]。这一过程中,植物所产生的活性氧副产物会损害细胞成分,迫使植物刺激抗氧化酶活性,从而刺激酚类化合物的合成,并建立一系列反应体系来应对活性氧的侵害[63-64]。因此,杂豆生长过程受灌溉、土壤、光照、施肥及其它人为因素的影响,导致酚类化合物的富集效果存在差异。一方面,土壤对于杂豆生长过程中酚类化合物合成有着较大作用,例如:土壤肥力及根瘤数量更佳的改良土壤对豇豆酚类化合物的富集具有促进作用[65],以及菌肥和果渣堆肥能够提高杂豆酚类化合物的含量[66-67]。另外,杀虫剂与化肥的缺乏使得有机杂豆更易受到生物胁迫的影响,导致杂豆中参与天然防御机制的酚类化合物的生成,进而导致总酚含量增加,例如:有机黑豆的总酚含量比非有机黑豆高28%[68]。另一方面,限制性灌溉和水分胁迫环境同样会导致菜豆酚类化合物含量的增加[64]。然而,环境因素发生变化的同时,不同种属杂豆的酚类化合物变化情况并非完全一致。当豌豆、蚕豆和扁豆暴露于不断上升的大气CO2 浓度时,CO2 浓度的升高将诱导蚕豆酚类化合物的积累,而对豌豆与扁豆的次级代谢物的水平没有影响[69],这可能涉及到不同种属杂豆的代谢调控机制差异。除此之外,受控条件下的人造光源对生长时期的杂豆酚类化合物的生物合成具有诱导作用,特别是蓝光可有效诱导花青素生物合成[70]。因此,环境变量影响杂豆发育阶段的酚类化合物生物合成,需要对变量进行精细控制,这将有助于在受控的农业生产环境中富集更多酚类化合物。
贮藏环境对成熟杂豆酚类化合物的含量与组成至关重要。贮藏期间的各种环境条件,如温度、湿度和光源等,会影响杂豆的代谢激活与酚类的生物合成途径[71]。首先,环境氧所引起的氧化过程是导致杂豆变色的主要因素[72],而变色过程涉及到酚类化合物的氧化降解,尤其是单宁和花青素类。其次,高温加速贮藏杂豆种皮颜色的加深,同时也将引起杂豆种皮及子叶化学成分的变化,并且酚类化合物的聚合及高温氧化降解是酚类化合物减少的重要原因[73],甚至低温冷藏或真空包装仍然无法避免贮藏菜豆总酚含量的下降,然而可减缓下降趋势[74]。另外,光源的存在同样会导致贮藏杂豆总酚含量下降,而不同于温度的影响机制,光源只能影响杂豆种皮。在光源条件下储藏12 个月后,杂豆种皮总酚含量显著减少,而子叶的总酚含量无显著变化,这是因为种皮可以阻挡或过滤光源[73]。除此之外,较高的相对湿度环境下贮藏的菜豆豆皮变黑,且原花青素B 型二聚体、表儿茶素和山奈酚含量较少[71]。另外,当贮藏杂豆的细胞膜受到高水分胁迫时,其总酚含量下降,并且贮藏杂豆种皮颜色变深[75-76]。贮藏过程对杂豆酚类化合物的影响,除了杂豆表观性质的改变,还必须考虑贮藏过程的内源性酶对杂豆酚类化合物的影响。扁豆贮藏过程的酶促和非酶促反应使原花青素聚合,导致它们与细胞壁的成分交联,从而低聚物的可提取性降低,使得黄烷-3-醇和原花青素含量减少,而复杂酚类化合物降解成低分子质量酚类化合物,使得其酚酸和黄酮的含量有所增加[77]。另外,采后UV-C 辐射能破坏植物DNA,并间接刺激植物的防御机制,从而诱导绿豆的苯丙氨酸解氨酶活性增强,以此触发酚类生物合成通路[78]。总之,贮藏期间杂豆的总酚含量普遍呈下降趋势,合理地控制杂豆含水量、光照强度及环境温度,或采用真空包装和气调包装可减缓这一过程。
3.3 加工的影响
杂豆的加工涉及各种形式的处理,如浸泡、发芽、热处理和发酵等。如表4 所示,在加工过程中,各种条件会影响杂豆的总酚含量,内源性酚类化合物可能会损失,甚至失去原有的抗氧化活性。相反,加工处理可破坏杂豆细胞壁与细胞内部结构,导致部分酚类化合物的释放。因此研究不同加工方式对杂豆酚类化合物影响的系统性规律,对杂豆加工方法的改良与创新具有指导意义。
表4 加工对杂豆总酚含量的影响
Table 4 Effect of processing on total phenolic content of pulses

(续表4)

浸泡是一个软化杂豆种子细胞壁组织、改善烹饪效果的过程。一方面,浸泡处理使得杂豆的水溶性酚类化合物流失在浸泡水中,导致总酚含量出现不同程度的下降,并且与浸泡时间成正比[85]。杂豆酚类化合物损失在浸泡水中,而超过50%的酚类化合物仍保留在皮层和子叶中,且杂豆酚类化合物的流失程度依赖于品种,需要根据品种调整浸泡过程[86]。酚类化合物的损失需要归咎于酚类化合物在杂豆基质中的扩散作用,例如:浸泡过程促进与细胞壁紧密结合的一些酚类化合物(如芦丁、飞燕草素-3-葡萄糖苷和槲皮素)的溶出与释放[87]。另一方面,浸泡过程能破坏杂豆酚类化合物与大分子化合物(多糖或蛋白)的稳定结构,促使结合态酚类化合物变成游离态,例如:绿豆的咖啡酸变为游离态,并且总酚含量增加23.17%[79]。因此,保留杂豆烹饪过程中的浸泡水可作为改善杂豆健康特性的一种策略。
发芽是一个复杂的生化过程,涉及到化合物的分解与合成[88]。在杂豆发芽过程中,杂豆内源酶的激活改善了酚类化合物组成与含量。发芽过程的酶促作用使得发芽黑豆富集甲氧基化花青素[89],并且整个过程受多个外在因素影响,例如:超声[88]、温度[90]、光照[91]及时间[92]等。在杂豆发芽过程中,杂豆细胞壁的分解使得不溶性结合酚类化合物水解为可溶性结合形式,与此同时,转移酶的作用使得可溶性游离酚类化合物转化为可溶性结合形式,例如:以可溶性结合形式存在的阿福豆素、樱黄素及刺芒柄花素的含量增加[31]。除此之外,杂豆发芽过程的胁迫处理能激发以黄酮生物合成通路为介导的防御反应。不同诱导剂(如抗坏血酸、叶酸和谷氨酸等)能激活苯丙烷途径,从而导致黄烷-3-醇和花青素的减少,以及促进黄烷醇及其糖苷的合成[93]。需要注意的是,苯丙烷生物合成前体(苯丙氨酸和酪氨酸)是限制酚类生物合成的关键因素[94]。总的来说,合理控制杂豆发芽过程中的外在条件可作为富集酚类化合物的一种有效途径。
作为杂豆最常见的加工方式,热处理过程可将杂豆加工成即食食品。水热处理是家庭烹饪以及食品工业中应用最广泛的加工方法,可软化豆类的质地,并且有效地减少或灭活抗营养物质[95]。一方面,水热处理可以破坏杂豆的细胞壁结构,促使酚类化合物溶出,并且水热处理过程导致的酚类化合物损失似乎是不可避免的[81]。不仅如此,蒸煮压力和时间影响种皮、子叶和蒸煮水的酚类化合物组成分布。在较长的蒸煮时间下,扩散到蒸煮用水和子叶的酚类化合物,可形成酚类-蛋白质复合体[96]。此类稳定的分子复合物多以氢键和疏水键形式相连,而水热处理过程中的水解作用可以破坏其稳定结构,使酚类化合物游离出来[97]。另一方面,不同于常规水热处理,蒸汽爆破加工是在高温和高压条件下,通过瞬时释压促使红豆与绿豆酚类化合物溶出的一种加工方式,而爆破过程的高温作用将造成酚类化合物结构的裂解[98]。热处理过程可导致酚类化合物转化为新的化合物,例如:对高温非常敏感的花色素苷,加热后可转化为查尔酮[99]。综上所述,热处理可破坏杂豆细胞壁结构,促进酚类化合物的释放,然而需要避免热处理导致的酚类化合物转化和裂解。
发酵是传统生物加工方法之一,具有改善酚类化合物含量,提高其营养价值的潜力[100]。一方面,发酵过程能够释放游离酚醛缩合物,导致杂豆酚类化合物含量上升,并且乳酸菌可保护某些酚类化合物免受化学降解[101]。另一方面,发酵菌株或酵母对酚类化合物(如阿魏酸、对香豆酸或绿原酸)生物转化的过程(图3)涉及到脱羧酶与还原酶的作用,例如原儿茶酸脱羧后转化为儿茶酚。其次,发酵过程的内源性酶作用能够水解共轭形式的酚类化合物,如在解淀粉芽孢杆菌LSSE-62 发酵的鹰嘴豆中,微生物分泌的β-葡萄糖苷酶可水解共轭形式以释放酚类化合物,导致发酵后鹰嘴豆的总酚含量增加了222%[102]。植物乳杆菌发酵豇豆粉的过程,水解槲皮素糖苷的酶被激活,使得部分槲皮素糖苷(槲皮素-3-O-葡萄糖苷与槲皮素-3-O-半乳糖苷)水解为槲皮素[103]。另外,内源性酶的激活与发酵微生物的代谢机制有关。董英丽[104]发现两种不同乳酸菌(NM701 和S5-5)发酵红豆乳的酚类化合物含量随着发酵时间的变化情况不同,初步推测乳酸菌S5-5 没有相关酚类生物合成的内源性酶,并且生成可氧化原有酚类化合物的代谢产物。与上述研究结果不同的是,发酵过程中的酸性环境是酚类物质减少的重要原因,低pH 环境促使酚类化合物结构的重排与氢化物的生成[105]。因此,发酵有助于酚类化合物的释放及结构的变化,然而需要选择合适的菌株。
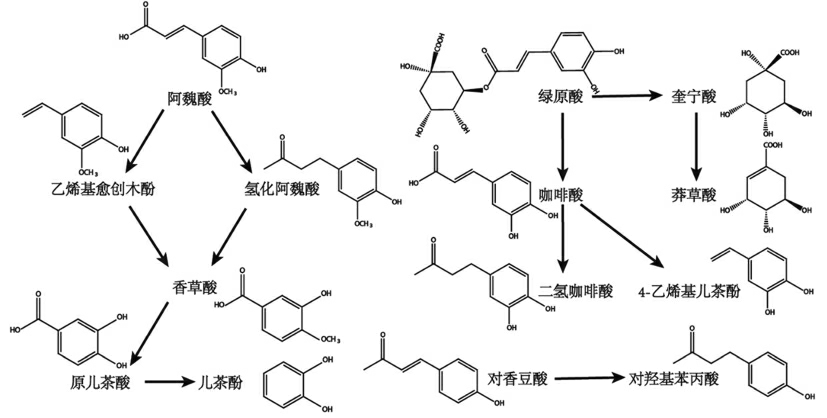
图3 发酵过程中酚酸可能涉及的生物转化过程[100]
Fig.3 Possible biotransformation processes involved in the fermentation of phenolic acids[100]
4 展望
基于上述影响杂豆酚类化合物多样性因素的概述,杂豆酚类化合物多样性受品种与产地、生长与贮藏及加工方式的影响,甚至是同时影响的,并且不同状态或条件对杂豆酚类化合物多样性的影响,涉及到酚类化合物的溶出、释放与生物合成变化。因此,尽快围绕杂豆构建酚类化合物多样性评价体系,以分析影响杂豆酚类化合物多样性的综合性因素,从而筛选并改良富含酚类化合物的杂豆种质。
杂豆酚类化合物多样性的研究,仍然存在不少亟待解决的问题。首先,在杂豆基因功能研究方面,尽管与酚类化合物的生物合成途径相关的大部分结构基因都已被确定,但对于合成途径的调控研究还处于早期阶段,缺乏对杂豆中调节基因的功能研究,进而阻碍了假定的酚类生物合成途径调节基因与基因功能的关联性分析。其次,杂豆的地方品种正在被可满足产量目标或其它特定需求的商业品种所取代,这将导致某些富含酚类化合物的地方品种基因库的丢失。另外,杂豆酚类化合物的提取方法多样化,没有一种通用的提取程序,需要依据样本类型及预期成分选择和调整提取方法,甚至在相同品种样本的前处理方法上还未能完全统一,这将影响杂豆酚类化合物的全面表征与多样性分析。最后,受样本共同栖息地与形态相似性影响,某些杂豆样本的不同品种之间会出现相互混杂的情况,造成采样困难且可用样本有限,进而影响杂豆酚类化合物多样性结果的准确性。与此同时,杂豆酚类化合物如何影响人类肠道菌群多样性及缓解氧化应激等相关健康功效,是杂豆酚类化合物功能和活性研究领域亟待解决的问题。杂豆酚类化合物功能活性受其多样性的影响,而影响机制是多方面的,需要构建酚类化合物多样性与活性效果的关联性。
多年来,酚类化合物的定性与定量方法得到快速发展与改进。一方面,部分仪器表征方法,例如高分辨质谱,可对杂豆酚类化合物进行快速的定量或定性分析,极大地提升了试验效率。另一方面,高灵敏度与分辨率的表征手段将有助于未知或复杂酚类化合物的表征,以应对杂豆样本的酚类化合物多样性的挑战。基于先进的化学表征方法,使用代谢组学技术可对杂豆样本的多维复杂性数据进行降维和信息挖掘,可监测杂豆酚类化合物的变化规律,有助于识别和量化限定条件下酚类化合物组成与含量的变化规律。随着代谢组学的发展,植物代谢组学已涉及杂豆酚类化合物合成机制和基因-环境的相互作用研究,从而挖掘杂豆酚类化合物多样性的原因。与传统方法相比,代谢组学规避了理化试验结果的单一性,可从全局出发,探索不同状态或条件下的差异单体酚类化合物。差异酚类化合物的发现为研究人员研究特定条件下杂豆酚类合成机制提供了基础。除此之外,多组学联用技术使得单一代谢组学的结果更加丰富,它的发展有助于杂豆样本从基因、蛋白及转录等角度综合分析,以解析杂豆酚类生物合成途径及其调控因子,特别是代谢组学与转录组学的联合分析,有助于确定参与特定代谢物生物合成的候选基因。
另外,注重采用组学技术研究与杂豆生长发育相关的代谢机制,并延伸至蛋白表达及基因修饰等领域,这有助于改良杂豆种植方法,从而富集更多酚类化合物及改善杂豆品质。这将为专注于提高总酚含量和探寻杂豆酚类化合物生物多样性提供帮助,这也为杂豆育种计划提供有用的信息。
[1] LÓPEZ -MARTÍNEZ L X,LEYVA -LÓPEZ N,GUTIÉRREZ -GRIJALVA E P,et al.Effect of cooking and germination on bioactive compounds in pulses and their health benefits[J].Journal of Functional Foods,2017,38:624-634.
[2] MARINANGELI C P F,CURRAN J,BARR S I,et al.Enhancing nutrition with pulses:Defining a recommended serving size for adults[J].Nutrition Reviews,2017,75(12):990-1006.
[3] LI L,YANG T,LIU R,et al.Food legume production in China[J].The Crop Journal,2017,5(2):115-126.
[4] MORENO-GARCÍA K L,ANTUNES-RICARDO M,MARTÍNEZ-ÁVILA M,et al.Evaluation of the antioxidant,anti -inflammatory and antihyperglycemic activities of black bean(Phaseolus vulgaris L.)byproduct extracts obtained by supercritical CO2[J].The Journal of Supercritical Fluids,2022,183:105560.
[5] PEREZ -HERNANDEZ L M,HERNÁNDEZ -ÁLVAREZ A J,MORGAN M,et al.Polyphenol bioaccessibility and anti -inflammatory activity of Mexican common beans(Phaseolus vulgaris L.)with diverse seed colour [J].CyTA -Journal of Food,2021,19(1):682-690.
[6] FONSECA-HERNANDEZ D,CERVANTES E D C L,MOJICA L.Bioactive compounds in fermented chickpeas and common beans [M].Washington:American Chemical Society,2022:115-133.
[7] LIU H K,CHEN Y Y,HU T T,et al.The influence of light-emitting diodes on the phenolic compounds and antioxidant activities in pea sprouts[J].Journal of Functional Foods,2016,25:459-465.
[8] YANG Q Q,GAN R Y,GE Y Y,et al.Polyphenols in common beans(Phaseolus vulgaris L.):Chemistry,analysis,and factors affecting composition[J].Comprehensive Reviews in Food Science Food Safety,2018,17(6):1518-1539.
[9] 李文婷,李红艳,邓泽元,等.基于UPLC-QTOFMS 和HPLC-QQQ-MS 技术的鹰嘴豆酚类化合物定性、定量分析及其抗氧化能力研究[J].中国食品学报,2020,20(4):261-269.LI W T,LI H Y,DENG Z Y,et al.Qualitative,quantitative analysis of chickpea phenolics based on UPLC-QTOF-MS and HPLC-QQQ-MS and It's the antioxidant activity[J].Journal of Chinese Institute of Food Science and Technology,2020,20(4):261-269.
[10] 王何柱,朱勇,朱怡,等.不同花色芸豆种皮酚类化合物组成及抗氧化活性[J].食品科学,2020,41(12):204-210.WANG H Z,ZHU Y,ZHU Y,et al.Phenolic composition and antioxidant activity of seed coats of kidney beans with different colors[J].Food Science,2020,41(12):204-210.
[11] 裴敏佳,王晓雅,熊华,等.基于超高效液相串联飞行时间质谱技术分析小扁豆豆壳多酚组成及其抗氧化活性[J].南昌大学学报(理科版),2021,45(3):265-272.PEI M J,WANG X Y,XIONG H,et al.Analysis of lentil hulls phenolics and antioxidant capacity based on UPLC-ESI-QTOF-MS2[J].Journal of Nanchang University(Natural Science),2021,45(3):265-272.
[12] YANG Q Q,YING G Y,ANIL G,et al.Phenolic profiles,antioxidant activities,and antiproliferative activities of different mung bean(Vigna radiata)varieties from Sri Lanka[J].Food Bioscience,2020,37:100705.
[13] DUEÑAS M,ESTRELLA I,HERNÁNDEZ T.Occurrence of phenolic compounds in the seed coat and the cotyledon of peas(Pisum sativum L.)[J].European Food Research &Technology,2004,219(2):116-123.
[14] MADRERA R R,VALLES B S.Development and validation of ultrasound assisted extraction(UAE)and HPLC -DAD method for determination of polyphenols in dry beans(Phaseolus vulgaris)[J].Journal of Food Composition and Analysis,2020,85:103334.
[15] IBÁEZ E.Phytochemical and functional characterization of phenolic compounds from cowpea(Vigna unguiculata(L.)Walp.)obtained by green extraction technologies[J].Agronomy,2021,11(1):162.
[16] 李文婷,顿倩,李红艳,等.基于UPLC-Q TOFMS 和HPLC-QQQ-MS 技术的赤豆酚类化合物定性、定量分析及其抗氧化能力分析[J].食品科学,2019,40(8):112-118.LI W T,DUN Q,LI H Y,et al.Qualitative and quantitative analysis of adzuki bean phenolics by ultra performance liquid chromatography quadrupole time-of-flight mass spectrometry and high performance liquid chromatography coupled with triple quadruple mass spectrometry and their antioxidant activity[J].Food Science,2019,40(8):112-118.
[17] XU B J,CHANG S K C.A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents[J].Journal of Food Science,2007,72(2):S159-S166.
[18] NAWAZ H,SHAD M A,REHMAN N,et al.Effect of solvent polarity on extraction yield and antioxidant properties of phytochemicals from bean(Phaseolus vulgaris)seeds[J].Brazilian Journal of Pharmaceutical Sciences,2020,56:e17129.
[19] NACZK M,SHAHIDI F.Extraction and analysis of phenolics in food[J].Journal of Chromatography A,2004,1054(1):95-111.
[20] SUTIVISEDSAK N,CHENG H N,WILLETT J L,et al.Microwave -assisted extraction of phenolics from bean(Phaseolus vulgaris L.)[J].Food Research International,2010,43(2):516-519.
[21] HERRERO M,PLAZA M,CIFUENTES A,et al.Extraction techniques for the determination of phenolic compounds in food[J].Comprehensive Sampling and Sample Preparation,2012,4:159-180.
[22] MACZ-POP G A,RIVAS-GONZALO J C,PÉREZALONSO J J,et al.Natural occurrence of free anthocyanin aglycones in beans(Phaseolus vulgaris L.)[J].Food Chemistry,2006,94(3):448-456.
[23] MADRERA R R,VALLES B S.Development and validation of ultrasound assisted extraction(UAE)and HPLC -DAD method for determination of polyphenols in dry beans(Phaseolus vulgaris)[J].Journal of Food Composition and Analysis,2020,85:103334.
[24] AMAROWICZ R,ESTRELLA I,HERNÁNDEZ T,et al.Antioxidant activity of a red lentil extract and its fractions[J].International Journal of Molecular Sciences,2009,10(12):5513-5527.
[25] YAO Y,CHENG X Z,WANG L X,et al.Major phenolic compounds,antioxidant capacity and antidiabetic potential of rice bean(Vigna umbellata L.)in China[J].International Journal of Molecular Sciences,2012,13(3):2707-2716.
[26] LIU R,CAI Z W,XU B J.Characterization and quantification of flavonoids and saponins in adzuki bean(Vigna angularis L.)by HPLC-DAD-ESI-MSn analysis[J].Chemistry Central Journal,2017,11(1):93.
[27] ZHENG Y T,LIU S,XIE J H,et al.Antioxidant,α-amylase and α-glucosidase inhibitory activities of bound polyphenols extracted from mung bean skin dietary fiber[J].LWT-Food Science and Technology,2020,132:109943.
[28] GAI Q Y,JIAO J,WANG X,et al.Simultaneous quantification of eleven bioactive phenolic compounds in pigeon pea natural resources and in vitro cultures by ultra-high performance liquid chromatography coupled with triple quadrupole mass spectrometry(UPLC-QqQ-MS/MS)[J].Food Chemistry,2021,335:127602.
[29] TEIXEIRA-GUEDES C I,OPPOLZER D,BARROS A I,et al.Impact of cooking method on phenolic composition and antioxidant potential of four varieties of Phaseolus vulgaris L.and Glycine max L [J].LWT-Food Science and Technology,2019,103:238-246.
[30] AGUILERA Y,DUEÑAS M,ESTRELLA I,et al.Phenolic profile and antioxidant capacity of chickpeas(Cicer arietinum L.)as affected by a dehydration process[J].Plant Foods for Human Nutrition,2011,66(2):187-195.
[31] XU M W,JIN Z,OHM J B,et al.Improvement of the antioxidative activity of soluble phenolic compounds in chickpea by germination[J].Journal of Agricultural and Food Chemistry,2018,66(24):6179-6187.
[32] DUEÑAS M,ESTRELLA I,HERNÁNDEZ T.Occurrence of phenolic compounds in the seed coat and the cotyledon of peas(Pisum sativum L.)[J].European Food Research and Technology,2004,219(2):116-123.
[33] MEENU M,KAMBOJ U,SHARMA A,et al.Green method for determination of phenolic compounds in mung bean(Vigna radiata L.)based on near-infrared spectroscopy and chemometrics[J].International Journal of Food Science &Technology,2016,51(12):2520-2527.
[34] APARICIO -FERNANDEZ X,YOUSEF G G,LOARCA -PINA G,et al.Characterization of polyphenolics in the seed coat of black Jamapa bean(Phaseolus vulgaris L.)[J].Journal of Agricul tural and Food Chemistry,2005,53(11):4615-4622.
[35] PIOVESANA S,CAVALIERE C,CERRATO A,et al.Developments and pitfalls in the characterization of phenolic compounds in food:From targeted analysis to metabolomics-based approaches[J].TrACTrends in Analytical Chemistry,2020,133:116083.
[36] SÁNCHEZ-RANGEL J C,BENAVIDES J,HEREDIA J B,et al.The Folin-Ciocalteu assay revisited:Improvement of its specificity for total phenolic content determination[J].Analytical Methods,2013,5(21):5990-5999.
[37] KUMAR B R.Application of HPLC and ESI-MS techniques in the analysis of phenolic acids and flavonoids from green leafy vegetables(GLVs)[J].Journal of Pharmaceutical Analysis,2017,7(6):349-364.
[38] HUANG Z L,WANG B W,EAVES D H,et al.Phenolic compound profile of selected vegetables frequently consumed by African Americans in the southeast United States[J].Food Chemistry,2007,103(4):1395-1402.
[39] CAPRIOLI G,NZEKOUE F K,GIUSTI F,et al.Optimization of an extraction method for the simultaneous quantification of sixteen polyphenols in thirty-one pulse samples by using HPLC-MS/MS dynamic-MRM triple quadrupole[J].Food Chemistry,2018,266:490-497.
[40] MYERS J R,WALLACE L T,MOGHADDAM S M,et al.Improving the health benefits of snap bean:Genome-wide association studies of total phenolic content[J].Nutrients,2019,11(10):2509.
[41] KAJIWARA V,MODA-CIRINO V,DOS SANTOS SCHOLZ M B.Studies on nutritional and functional properties of various genotypes of Andean beans[J].Journal of Food Science and Technology,2022,59(4):1468-1477.
[42] VANDENBERG A,SLINKARD A E.Genetics of seed coat color and pattern in lentil[J].Journal of Heredity,1990,81(6):484-488.
[43] MAHLA M,RANDY W P,ALBERT V,et al.Profiling the phenolic compounds of the four major seed coat types and their relation to color genes in lentil[J].Journal of Natural Products,2017,80(5):1310-1317.
[44] RODRÍGUEZ M R,CAMPA N A,SUÁREZ V B,et al.Characterization of extractable phenolic profile of common bean seeds(Phaseolus vulgaris L.)in a Spanish diversity panel[J].Food Research International,2020,138:109713.
[45] SICA P,GALVAO A,SCARIOLO F,et al.Effects of drought on yield and nutraceutical properties of beans(Phaseolus spp.)traditionally cultivated in Veneto,Italy[J].Horticulturae,2021,7(17):1-15.
[46] GARCÍA -DÍAZ Y,AQUINO -BOLAOS E N,CHÁVEZ-SERVIA J,et al.Bioactive compounds and antioxidant activity in the common bean are influenced by crop season and genotype[J].Chilean Journal of Agricultural Research,2018,78(2):255-265.
[47] CARBAS B,MACHADO N,OPPOLZER D,et al.Nutrients,antinutrients,phenolic composition,and antioxidant activity of common bean cultivars and their potential for food applications[J].Antioxidants,2020,9(2):186.
[48] 王何柱,朱勇,朱怡,等.7 种芸豆中酚类化合物组成及其抗氧化活性[J].中国粮油学报,2020,35(9):28-33.WANG H Z,ZHU Y,ZHU Y,et al.Composition and antioxidant activity of phenolic compounds in seven Kidney beans[J].Journal of the Chinese Cereals and Oils Association,2020,35(9):28-33.
[49] NITHIYANANTHAM S,SELVAKUMAR S,SIDDHURAJU P.Total phenolic content and antioxidant activity of two different solvent extracts from raw and processed legumes,Cicer arietinum L.and Pisum sativum L[J].Journal of Food Composition and Analysis,2012,27(1):52-60.
[50] 赵建京,成珊,范志红,等.我国不同产地绿豆抗氧化活性分析[J].食品工业科技,2010,31(11):144-147.ZHAO J J,CHENG S,FAN Z H,et al.Analysis of antioxidant capacity of mung beans from different areas in China[J].Science and Technology of Food Industry,2010,31(11):144-147.
[51] DEVI J,SANWAL S K,KOLEY T K,et al.Variations in the total phenolics and antioxidant activities among garden pea(Pisum sativum L.)genotypes differing for maturity duration,seed and flower traits and their association with the yield[J].Scientia Horticulturae,2019,244:141-150.
[52] BAGINSKY C,PEÑA-NEIRA Á,CÁCERES A,et al.Phenolic compound composition in immature seeds of fava bean(Vicia faba L.)varieties cultivated in Chile[J].Journal of Food Composition and Analysis,2013,31(1):1-6.
[53] SHETTY P,ATALLAH M T,SHETTY K.Effects of UV treatment on the proline-linked pentose phosphate pathway for phenolics and L-DOPA synthesis in dark germinated Vicia faba[J].Process Biochemistry,2002,37(11):1285-1295.
[54] HASSAN I A,BASAHI J M,KADI M W,et al.Physiological and biochemical impairment in bean plants due to supplementary ultraviolet radiation and water stress:Possible protective roles of secondary metabolites[J].Australian Journal of Basic and Applied Sciences,2012,6(9):552-561.
[55] DORIA E,CAMPION B,SPARVOLI F,et al.Anti-nutrient components and metabolites with health implications in seeds of 10 common bean(Phaseolus vulgaris L.and Phaseolus lunatus L.)landraces cultivated in southern Italy[J].Journal of Food Composition and Analysis,2012,26(1):72-80.
[56] ZHAO T Y,SU W J,QIN Y,et al.Phenotypic diversity of pea(Pisum sativum L.)varieties and the polyphenols,flavonoids,and antioxidant activity of their seeds[J].Ciência Rural,2020,50(5):e20190196.
[57] IRAKLI M,KARGIOTIDOU A,TIGKA E,et al.Genotypic and environmental effect on the concentration of phytochemical contents of lentil(Lens culinaris L.)[J].Agronomy,2021,11(6):1154.
[58] NICOLETTO C,ZANIN G,SAMBO P,et al.Quality assessment of typical common bean genotypes cultivated in temperate climate conditions and different growth locations[J].Scientia Horticulturae,2019,256:108599.
[59] WANG J J,LIU H,REN G X.Near-infrared spectroscopy(NIRS)evaluation and regional analysis of Chinese faba bean(Vicia faba L.)[J].The Crop Journal,2014,2(1):28-37.
[60] YAO Y,CHENG X Z,WANG S H,et al.Influence of altitudinal variation on the antioxidant and antidiabetic potential of azuki bean(Vigna angularis)[J].International Journal of Food Sciences and Nutrition,2012,63(1):117-124.
[61] GERSHENZON J.Secondary metabolites and plant defense[J].Plant Physiology,2002,3:283-308.
[62] DOS SANTOS T B,BUDZINSKI I G F,MARUR,C J,et al.Expression of three galactinol synthase isoforms in Coffea arabica L.and accumulation of raffinose and stachyose in response to abiotic stresses[J].Plant Physiology and Biochemistry,2011,49(4):441-448.
[63] SAIRAM R K,TYAGI A.Physiology and molecular biology of salinity stress tolerance in plants[J].Current Science,2004,86(3):157-186.
[64] HERRERA M D,REYNOSO -CAMACHO R,MELERO-MERAZ V,et al.Impact of soil moisture on common bean(Phaseolus vulgaris L.)phytochemicals[J].Journal of Food Composition and Analysis,2021,99:103883.
[65] PHARES C A,ATIAH K,FRIMPONG K A,et al.Application of biochar and inorganic phosphorus fertilizer influenced rhizosphere soil characteristics,nodule formation and phytoconstituents of cowpea grown on tropical soil[J].Heliyon,2020,6(10):e05255.
[66] CUCCI G,LACOLLA G,SUMMO C,et al.Effect of organic and mineral fertilization on faba bean(Vicia faba L.)[J].Scientia Horticulturae,2019,243:338-343.
[67] NAMAZI Y,REZAEI-CHIYANEH E,SIAVASH M S,et al.The effects of microbial inoculation and intercropping on yield and active ingredients of savory(Satureja hortensis L.)intercropped with common bean(Phaseolus vulgaris L.)[J].International Journal of Environmental Science and Technology,2022,19(9):8273-8288.
[68] BARRETO N M B,PIMENTA N G,BRAZ B F,et al.Organic black beans(Phaseolus vulgaris L.)from rio de janeiro state,brazil,present more phenolic compounds and better nutritional profile than nonorganic[J].Foods,2021,10(4):900.
[69] GARMENDIA I,RASHIDI S,QUEZADA-SALIRROSAS M R A,et al.Atmospheric CO2 concentration affects the life cycle,yield,and fruit quality of early maturing edible legume cultivars[J].Journal of the Science of Food and Agriculture,2021,102(10):3964-3971.
[70] KADOMURA-ISHIKAWA Y,MIYAWAKI K,NOJI S,et al.Phototropin 2 is involved in blue light-induced anthocyanin accumulation in Fragaria x ananassa fruits[J].Journal of Plant Research,2013,126:847-857.
[71] COELHO S R M,ALVES FILHO E G,SILVA L M A,et al.NMR and LC-MS assessment of compound variability of common bean(Phaseolus vulgaris)stored under controlled atmosphere[J].LWTFood Science and Technology,2020,117:108673.
[72] STANLEY D W.Hard beans-a problem for growers,processors,and consumers[J].Horttechnology,1992,2(3):370-378.
[73] NASAR-ABBAS S M,SIDDIQUE K H M,PLUMMER J A,et al.Faba bean(Vicia faba L.)seeds darken rapidly and phenolic content falls when stored at higher temperature,moisture and light intensity [J].LWT -Food Science and Technology,2009,42(10):1703-1711.
[74] ÁVILA B P,NORA F R,PINTO SEIXAS NETO A C,et al.Proteomic and physicochemical characteristics:The search for a quality profile of beans(Phaseolus vulgaris L.)during long-term storage[J].LWT-Food Science and Technology,2020,133:110057.
[75] KIBAR H,KIBAR B.Changes in some nutritional,bioactive and morpho -physiological properties of common bean depending on cold storage and seed moisture contents[J].Journal of Stored Products Research,2019,84:101531.
[76] RANI P R,CHELLADURAI V,JAYAS D S,et al.Storage studies on pinto beans under different moisture contents and temperature regimes[J].Journal of Stored Products Research,2013,52:78-85.
[77] MIRALI M,PURVES R W,VANDENBERG A.Phenolic profiling of green lentil(Lens culinaris Medic.)seeds subjected to long-term storage[J].European Food Research and Technology,2016,242(12):2161-2170.
[78] USTUN H,ALI Q,KURUBAS M S,et al.Influence of postharvest UV-C illumination on biochemical properties of green beans[J].Scientia Horticulturae,2021,289:110499.
[79] 刘婷婷,包佳微,李嘉欣,等.浸泡和发芽对杂豆酚类物质及其抗氧化性的影响[J].中国粮油学报,2019,34(8):26-33.LIU T T,BAO J W,LI J X,et al.Effects of soaking and germination on the content and antioxidant activity of phenols of beans[J].Journal of the Chinese Cereals and Oils Association,2019,34(8):26-33.
[80] ALKALTHAM M S,SALAMATULLAH A M,ÖZCAN M M,et al.The effects of germination and heating on bioactive properties,phenolic compounds and mineral contents of green gram seeds[J].LWTFood Science and Technology,2020,134:110106.
[81] LAFARGA T,VILLARÓ S,BOBO G,et al.Bioaccessibility and antioxidant activity of phenolic compounds in cooked pulses[J].International Journal of Food Science &Technology,2019,54(5):1816-1823.
[82] BORGES-MARTÍNEZ E,GALLARDO-VELÁZQUEZ T,CARDADOR -MARTÍNEZ A,et al.Phenolic compounds profile and antioxidant activity of pea(Pisum sativum L.)and black bean(Phaseolus vulgaris L.)sprouts[J].Food Science and Technology,2021,42:e45920.
[83] YADAV N,KAUR D,MALAVIYA R,et al.Effect of thermal and non-thermal processing on antioxidant potential of cowpea seeds[J].International Journal of Food Properties,2018,21(1):437-451.
[84] AREVALO I,GUZMÁN -MALDONADO S H,SANCHEZ S M M,et al.Steaming and toasting reduce the nutrimental quality,total phenols and antioxidant capacity of fresh kabuli chickpea(Cicer arietinum L.)[J].Plant Foods for Human Nutrition,2020,75(4):628-634.
[85] FERNANDES A C,NISHIDA W,DA COSTA PROENÇA R P.Influence of soaking on the nutritional quality of common beans(Phaseolus vulgaris L.)cooked with or without the soaking water:A review[J].International Journal of Food Science &Technology,2010,45(11):2209-2218.
[86] MECHA E,LEITÃO S T,CARBAS B,et al.Characterization of soaking process' impact in common beans phenolic composition:Contribute from the unexplored portuguese germplasm [J].Foods,2019,8(8):296.
[87] GIUSTI F,CAPUANO E,SAGRATINI G,et al.A comprehensive investigation of the behaviour of phenolic compounds in legumes during domestic cooking and in vitro digestion[J].Food Chemistry,2019,285:458-467.
[88] AMPOFO J O,NGADI M.Ultrasonic assisted phenolic elicitation and antioxidant potential of common bean(Phaseolus vulgaris)sprouts[J].Ultrasonics Sonochemistry,2020,64:104974.
[89] LÓPEZ A,EL-NAGGAR T,DUEÑAS M,et al.Effect of cooking and germination on phenolic composition and biological properties of dark beans(Phaseolus vulgaris L.)[J].Food Chemistry,2013,138(1):547-555.
[90] AMPOFO J O,NGADI M,RAMASWAMY H S.The impact of temperature treatments on elicitation of the phenylpropanoid pathway,phenolic accumulations and antioxidative capacities of common bean(Phaseolus vulgaris)sprouts[J].Food and Bioprocess Technology,2020,13(9):1544-1555.
[91] MASTROPASQUA L,DIPIERRO N,PACIOLLA C.Effects of darkness and light spectra on nutrients and pigments in radish,soybean,mung bean and pumpkin sprouts[J].Antioxidants,2020,9(6):558.
[92] ![]() ,GAWLIK-DZIKI U,KOWALCZYK D,et al.Impact of germination time and type of illumination on the antioxidant compounds and antioxidant capacity of Lens culinaris sprouts[J].Scientia Horticulturae,2012,140:87-95.
,GAWLIK-DZIKI U,KOWALCZYK D,et al.Impact of germination time and type of illumination on the antioxidant compounds and antioxidant capacity of Lens culinaris sprouts[J].Scientia Horticulturae,2012,140:87-95.
[93] DUEÑAS M,MARTÍNEZ -VILLALUENGA C,LIMÓN R I,et al.Effect of germination and elicitation on phenolic composition and bioactivity of kidney beans[J].Food Research International,2015,70:55-63.
[94] ![]() ,GAWLIK-DZIKI U.Elicitation and precursor feeding as tools for the improvement of the phenolic content and antioxidant activity of lentil sprouts[J].Food Chemistry,2014,161:288-295.
,GAWLIK-DZIKI U.Elicitation and precursor feeding as tools for the improvement of the phenolic content and antioxidant activity of lentil sprouts[J].Food Chemistry,2014,161:288-295.
[95] KHATTAB R Y,ARNTFIELD S D.Nutritional quality of legume seeds as affected by some physical treatments 2.Antinutritional factors[J].LWT-Food Science and Technology,2009,42(6):1113-1118.
[96] ROCHA-GUZMÁN N E,GONZÁLEZ-LAREDO R F,IBARRA-PÉREZ F J,et al.Effect of pressure cooking on the antioxidant activity of extracts from three common bean(Phaseolus vulgaris L.)cultivars[J].Food Chemistry,2007,100(1):31-35.
[97] 石碧,狄莹.植物多酚[M].北京:科学出版社,2002:206-207.SHI B,DI Y.Plant polyphenol[M].Beijing:Science Press,2002:206-207.
[98] 候春宇.蒸汽爆破加工对红豆和绿豆中不同结合态多酚及抗氧化活性的影响[D].长春:吉林农业大学,2020.HOU C Y.Effects of steam explosion on different bound polyphenols and antioxidant activity in red and mung beans[D].Changchun:Jilin Agricultural University,2020.
[99] MASTURA H Y,HASNAH H,DANG T.Total phenolic content and antioxidant capacity of beans:Organic vs inorganic[J].International Food Research Journal,2017,24(2):510.
[100] LEONARD W,ZHANG P,YING D,et al.Fermentation transforms the phenolic profiles and bioactivities of plant-based foods[J].Biotechnology Advances,2021,49:107763.
[101] SÁNCHEZ -MALDONADO A F,SCHIEBER A,GÄNZLE M G.Structure-function relationships of the antibacterial activity of phenolic acids and their metabolism by lactic acid bacteria[J].Journal of Applied Microbiology,2011,111(5):1176-1184.
[102] WEI X T,LUO M F,XU L,et al.Production of fibrinolytic enzyme from Bacillus amyloliquefaciens by fermentation of chickpeas,with the evaluation of the anticoagulant and antioxidant properties of chickpeas[J].Journal of Agricultural and Food Chemistry,2011,59(8):3957-3963.
[103] DUEÑAS M,FERNÁNDEZ D,HERNÁNDEZ T,et al.Bioactive phenolic compounds of cowpeas(Vigna sinensis L).Modifications by fermentation with natural microflora and with Lactobacillus plantarum ATCC 14917[J].Journal of the Science of Food and Agriculture,2005,85(2):297-304.
[104] 董英丽.乳酸菌对红豆发酵特性的研究[D].呼和浩特:内蒙古农业大学,2013.DONG Y L.Study on fermentation characteristics of Vigna unbellata by lactic acid bacteria[D].Hohhot:Inner Mongolia Agricultural University,2013.
[105] TOWO E,MATUSCHEK E,SVANBERG U.Fermentation and enzyme treatment of tannin sorghum gruels:Effects on phenolic compounds,phytate and in vitro accessible iron[J].Food Chemistry,2006,94(3):369-376.